Dho 225
CM-anom or ung
Possible ‘CY’ ChondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More
(thermally metamorphosed/dehydrated)
Found January 15, 2001
18° 21.6′ N., 54° 11.3′ E. A fresh, black, carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More weighing just 90 g was found in the desert of Oman. Dhofar 225 has textural characteristics similar to typical CM chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, but differs from members of that group in mineralogy, bulk composition, and O-isotopic composition (Ivanova et al., 2010). The chromium oxide content of Dhofar 225 indicates a petrologic typeMeasure of the degree of aqueous alteration (Types 1 and 2) and thermal metamorphism (Types 3-6) experienced by a chondritic meteorite. Type 3 chondrites are further subdivided into 3.0 through 3.9 subtypes. below 3.0.
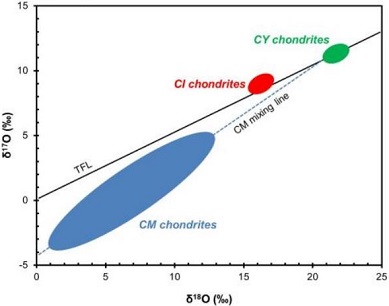
Diagram credit: King and Russell, 50th LPSC, #1386 (2019) Other possibly related dehydrated CM-like meteorites include Y-86789 (C2-ung, likely paired with Y-86720), WIS 91600 (CM2), EET 96010 (CM2), PCA 02012 (CM2). In addition, two meteorites classified as CI, Y-86029 and Y-980115, were determined by King and Russell (2019) to have similar mineralogical and chemical similarities to the CY group. In addition, Nakamura (2006) identified two regolithMixture of unconsolidated rocky fragments, soil, dust and other fine granular particles blanketing the surface of a body lacking an atmosphere. Regolith is the product of "gardening" by repeated meteorite impacts, and thermal processes (such as repeated heating and cooling cycles). Click on Term to Read More breccias containing solar-wind-implanted noble gasesElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More which belong to this dehydrated group, Y-793321 (CM2) and A-881458 (CM2), while M.M.M. Meier (2014) found that the meteorite Diepenveen (CM2-an) also contains similar trapped solar gases. Another ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More C2 meteorite with a similar O-isotopic composition is Dhofar 1988 (oxygen isotope plot; photo [courtesy of Marcin Cimala]), which was found by M. Cimala in 2011. In addition, the ungrouped C chondrite Dhofar 2066 also has a heavy oxygen isotope composition similar to the CY group (oxygen isotope plot) as do Y-86737 and Y-980134 which are both classified as CI1 (King and Russell, 2019). Notably, Dhofar 225 has many features and an oxygen isotopic composition similar to the anomalous CM chondriteClass of carbonaceous chondrites named after the Mighei meteorite that fell in Ukraine in 1889. They represent samples of incompletely serpentinized primitive asteroids and have experience extremely complex histories. CM meteorites are generally petrologic level type 2 though a few examples of CM1 and CM1/2 also exist. Compared to CI Click on Term to Read More Dhofar 735 (oxygen isotope plot; photo), which along with Belgica 7904 and PCA 02012, have experienced the highest temperatures (~900°C) over a brief time interval (PCA 02012 estimated at tens of hours; Nakato et al., 2013) compared to other members belonging to the CY group. The case for a distinct CY group as proposed by Y. Ikeda (1992) is strengthened by a mineralogical comparison conducted by King and Russell (2019). The significantly higher modal sulfide content in the CY-group chondrites Y-980115 and Y-82162 compared to that of average CM and CI chondrites is difficult to reconcile with an attribution to hydrationReaction of a substance with water. Click on Term to Read More/dehydration processes, but is instead more consistent with a difference in primary mineralogy (see diagram below).
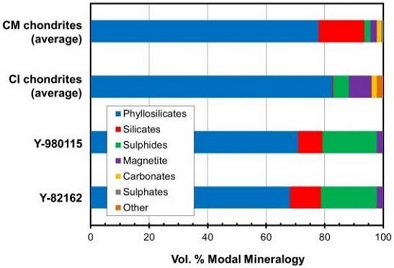
Diagram credit: King and Russell, 50th LPSC, #1386 (2019) Differences exist between Dhofar 225 and Dhofar 735 on one hand, and the Belgica-like grouplet on the other. In contrast to the FeNi-metal grains present among Belgica-like meteorites, those in Dhofar 225 and Dhofar 735 are not enriched in Cr and P (Ivanova et al., 2010). Moreover, the bulk chemistry between the Dhofar and Belgica metamorphosed meteorites are different. Similar to the Belgica grouplet, but unlike typical CM chondrites, Dhofar 225 exhibits considerable but incomplete dehydration of matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More phyllosilicatesClass of hydroxyl-bearing silicate minerals with a sheet-like structure. They result from aqueous alteration are dominantly serpentine and smectite in meteorites; found in the matrixes of carbonaceous chondrites. Phyllosilicates consist of repeating sequences of sheets of linked tetrahedra (T) and sheets of linked octahedra (O). The T sheet consists of Click on Term to Read More (<2 wt% water), Fe and S depletions, and contains tiny grains of tetrataenite within the matrix—all features consistent with a higher thermal metamorphism than that experienced by typical CM group members. However, sharp zoning profiles of olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More in the chondrule-like objects of Dhofar 225 severely constrain the maximum temperature of metamorphism. In particular, zoning of olivine grains observed in Dhofar 735 and Belgica 7904 indicates a short heating duration that negates the theory of heating by decay of radioactive elements (Nakato et al., 2011). Aqueously altered carbonaceous chondrites that have experienced thermal metamorphism have been classified according to their degree of heating and corresponding phyllosilicate dehydration. Estimates of dehydration temperatures are shown below (Nakamura, 2005):
| Dehydration Temperature | |
|---|---|
| Stage I | <300°C |
| Stage II | 300–500°C |
| Stage III | 500–750°C |
| Stage IV | >750°C |
ChondrulesRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More in Dhofar 225 are sparse (24 vol%), and similar in size (0.3 mm) to those of CM chondrites. Olivine is forsteritic and commonly occurs as aggregates up to 0.6 mm in size, and as chondrule-like objects. The matrix constitutes 70 vol% and is primarily composed of phyllosilicates (serpentineName used for a large group of phyllosilicate minerals with the generalized formula X2-3 Y2 O5 (OH)4. Due to their various structures (meteoritics focuses primarily on (Fe, Mg)3Si2O5(OH)4), serpentine can be used to understand the chemistry and progress of aqueous alteration (hydration) of olivine, amphibole, or pyroxene dating back to Click on Term to Read More), with minor sulfides, phosphides, phosphates, FeNi-metal, and chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More, with only rare CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More (2 vol%). A previously unknown mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More phase, Ca,Fe-oxysulfide, was identified in the matrix, possibly an oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More product of a primary sulfide phase (Ivanova et al., 2010). Tochilinite, characteristically abundant in CM chondrites, has been mostly thermally decomposed to troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More and oxides in both Dhofar 225 and Dhofar 735, as well as in the Belgica-like grouplet. The similarly thermally unstable P-rich oxysulfides only occur in very low abundances (Ivanova et al., 2005). Other rare minerals identified include eskolaite and Cr-barringerite.
In contrast to the low-Ni, low-Co content of the metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More within chondrules of Dhofar 225, the composition of the matrix metal is high-Ni, high-Co taeniteLess common than kamacite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Taenite, γ-(Fe,Ni), has 27-65 wt% Ni, and forms small crystals that appear as highly reflecting thin ribbons on the etched surface of a meteorite; the name derives from the Greek word for "ribbon." Click on Term to Read More and tetrataenite. The Fe/Si matrix ratio of Dhofar 225 is consistent with that of the CM chondrite group. The absence of Cr and P in the metal of Dhofar 225 is similar to that in the metamorphosed meteorites Belgica 7904 and Y-86720. Although the matrix of Dhofar 225 is compositionally similar to CI chondrites, especially Y-82162, as well as to the metamorphosed-CM chondrite Y-86720, only Dhofar 225 has retained moderate abundances of tochilinite-cronstedtite intergrowths (TCI; formerly PCP or ‘poorly characterized phases’). This specific mineralogy suggests that the grouplet experienced a period of variable aqueous alteration followed by a low level heating/dehydration phase, probably caused by impacts (Choe et al., 2010). A later episode of aqueous alteration might have affected Dhofar 225 resulting in its extant tochilinite. While this group of metamorphosed carbonaceous chondrites could have been derived from normal CM chondrites, in accord with their many common characterisics, some researchers consider it more likely that they originated from one or more separate parent bodies. This scenario can explain the significant difference in O-isotopic compositions between the metamorphosed Dhofar meteorites (and the Belgica-like grouplet) and typical CM chondrites (Choe et al., 2010). Furthermore, geochemical variations that exist between the two Dhofar meteorites and the Belgica meteorites attest to the fact that their source material was not exactly the same. It was accepted that these metamorphosed meteorites could not have been derived from typical CM2 material through dehydration processes, but rather were formed in a similar O-reservoir (Ivanova et al., 2010). Continued research by Ivanova et al. (2012, 2013) has demonstrated that the differences observed in the O-isotopic composition between the metamorphosed carbonaceous chondrites of the Dhofar and Belgica-like groupings and typical CM chondrites are consistent with multiple cycles of hydration–dehydration on a common parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More. Following aqueous alteration of silicates involving a source of water enriched in 18O, the resulting phyllosilicate (primarily serpentine) was also enriched in 18O by ~10%. Moreover, subsequent dehydration processes led to a further enrichment in 18O by ~7%. They reasoned that a low degree of heating at some distance from an impact craterCrater formed by high-speed impact of a meteoroid, asteroid, or comet on a solid surface. Craters are a common feature on most moons (an exception is Io), asteroids, and rocky planets, and range in size from a few cm to over 1,000 km across. There is a general morphological progression Click on Term to Read More would result in melting of extant water ice, which was then utilized in the hydration of silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the rock—then followed burial, metamorphism, and dehydration of this rock. They propose that this hydration–dehydration cycle may have occurred multiple times to produce the isotopic and geochemical differences observed among these meteorites. Heating experiments were conducted by Nakato et al. (2014, 2016) in which samples of the C2-ungrouped Tagish Lake, a meteorite that shares many characteristics with metamorphosed carbonaceous chondrites, were exposed to a varying temperatures and heating durations. They demonstrated that heating at a high temperature of 900°C for 1–96 hours caused progressive reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More and dehydration, resulting in mineralogical and textural changes similar to those observed in the Belgica group of thermally metamorphosed carbonaceous chondrites (e.g., fibrous textured phyllosilicates, reduction of magnetiteFe oxide, Fe2+Fe3+2O4, containing oxidized iron (Fe3+) found in the matrix of carbonaceous chondrites and as diagnostic component in CK chondrites. In CK chondrites, magnetite is typically chromian, containing several wt. % Cr2O3. Click on Term to Read More to form FeNi–metal+troilite assemblages). In addition, both Tagish Lake and the Belgica group meteorites have Si-rich matrix compositions compared to typical low-temperature CM chondrites. The degree of change in the O-isotopic composition of these heated samples is yet to be established. Current studies suggest that both cometary dust and meteorites should be produced from the disruption of Jupiter-family comets which originate in the Kuiper beltRegion in the outer solar system beyond Neptune's orbit that contains billions of small, icy planetesimals from the original protoplanetary disc that failed to coalesce into planets. The Kuiper Belt extends from Neptune's orbit at 30 AU to ~55 AU. It is ~20x wider and 20-200x more massive than the Click on Term to Read More. Studies have shown that Antarctic micrometeorites have a similar carbonaceous chondrite:ordinary chondriteWork in Progress Ordinary chondrites (OCs) are the largest meteorite clan, comprising approximately 87% of the global collection and 78% of all falls (Meteoritical Society database 2018)1. Meteorites & the Early Solar System: page 581 section 6.1 OC of type 5 or 6 with an apparent shock stage of S1, Click on Term to Read More ratio (~7:1) as the composition of zodiacal dust (M.M.M. Meier, 2014). Based on observational evidence and current modeling, it is thought that comets should be dark in color and have a low densityMass of an object divided by its volume. Density is a characteristic property of a substance (rock vs. ice, e.g.). Some substances (like gases) are easily compressible and have different densities depending on how much pressure is exerted upon them. The Sun is composed of compressible gases and is much Click on Term to Read More and strength, a high porosityThe volume percentage of a rock that consists of void space. Vesicular porosity is a type of porosity resulting from the presence of vesicles, or gas bubbles, in igneous rock such as the pumice presented here. Vesicular porosity is very rare in meteorites and is often associated with slag, one Click on Term to Read More, a solar ratio of elements, an elevated ratio of C, H, O, and N, a high interstellar grain content, anhydrous and highly unequilibrated silicates, few to no chondrules, and a low cosmic-ray exposure ageTime interval that a meteoroid was an independent body in space. In other words, the time between when a meteoroid was broken off its parent body and its arrival on Earth as a meteorite - also known simply as the "exposure age." It can be estimated from the observed effects Click on Term to Read More (<10 m.y.). Both the CI and CM groups of meteorites exhibit characteristics which are consistent with the above descriptions. Orbital data obtained from several carbonaceous chondrites (e.g., CI OrgueilA large carbonaceous Ivuna-like (CI1) chondrite that disintegrated and fell in fragments near the French town of Orgueil on May 14, 1864. About 20 pieces, totaling ~12 kg in mass, were subsequently recovered from an area of several square km, some head-sized but most were smaller than a fist. Specimens Click on Term to Read More [eyewitness plotting]; CMs Maribo and Sutter’s Mill [instrument recording]) are a good match to the orbits expected from the disruption of Jupiter-family comets, but are unlike the orbits of ordinary chondrites and most other asteroidal objects (M.M.M. Meier, 2014). Both the orbital eccentricityThe deviation of an orbit from circularity. Circles have eccentricities of 0. Click on Term to Read More and semimajor axis for Maribo is nearly identical to those of CometConglomeration of frozen water and gases (methane, ammonia, CO2) and silicates that that formed in the outer solar system and orbits the Sun. In recent years, the description of comets has shifted from dirty snowballs to snowy dirtballs with more dust than ice. However, the ratio is less than 10-to-1. Click on Term to Read More Encke and the associated Taurid swarm of objects (Haack et al., 2011). On the other hand, a CRE age study of CM chondrites conducted by Meier et al. (2016) shows a possible relationship exists to the asteroid breakup event ~8.3 m.y. ago that formed the Ch/C/Cg-type members of the Veritas family. In addition to the large abundance of 3He-enriched interplanetary dust discovered in 8.2 m.y.-old deep-sea drill cores, ~1/6 of all CM meteorites have 21Ne-based CRE ages that are consistent with derivation from this catastrophic breakup, while others with significantly younger CRE ages could represent secondary collisions among the Veritas fragments. In consideration of the young CRE age of all of the Belgica group meteorites, a near-Earth asteroidAsteroids with orbits that bring them within 1.3 AU (195 million km) of the Sun. NEAs are a dynamically young population whose orbits evolve on 100-million-year time scales because of collisions and gravitational interactions with the Sun and the terrestrial planets. These asteroids are probably ejected from the main belt Click on Term to Read More is favored as the common source object. One possible candidate is the binary asteroid 1998 ST27, which appears to match the required spectrographic characterisics of these meteorites. Moreover, its binary nature is consistent with the likelihood for disruption and injection of material into an Earth approaching orbitThe elliptical path of one body around another, typically the path of a small body around a much larger body. However, depending on the mass distribution of the objects, they may rotate around an empty spot in space • The Moon orbits around the Earth. • The Earth orbits around Click on Term to Read More. Other source asteroids, such as Phaethon, Icarus, and 2008 FF5, are considered by Ivanova et al. (2013) as potential sources for these meteorites; i.e., the heat source for their metamorphism may be associated with their perihelion close to the SunOur parent star. The structure of Sun's interior is the result of the hydrostatic equilibrium between gravity and the pressure of the gas. The interior consists of three shells: the core, radiative region, and convective region. Image source: http://eclipse99.nasa.gov/pages/SunActiv.html. The core is the hot, dense central region in which the. Notably, the C-type asteroid 162173 Ryugu, from which a sample return is planned for 2020 by the spacecraft Hayabusa2, has some spectral similarity to experimentally-heated hydrated carbonaceous chondrites which may be analogous to those of the CY group (Matsuoka et al., 2018; King and Russell, 2019). The specimen of Dhofar 225 shown above is a 0.69 g partial end section.






