Serpentine
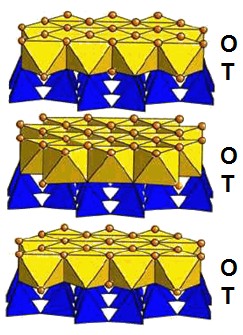
Name used for a large group of phyllosilicate minerals with the generalized formula X2-3 Y2 O5 (OH)4. Due to their various structures (meteoriticsScience involved in the study of meteorites and related materials. Meteoritics are closely connected to cosmochemistry, mineralogy and geochemistry. A scientist that specializes in meteoritics is called a meteoriticist. Click on Term to Read More focuses primarily on (Fe, Mg)3Si2O5(OH)4), serpentine can be used to understand the chemistry and progress of aqueous alteration (hydrationReaction of a substance with water. Click on Term to Read More) of olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More, amphibole, or pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More dating back to the formation of the solar systemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids.. The hydration process is called “serpentinization”. When serpentinization occurs with other geochemical mechanisms, the process can produce a broad range of abioticNon-biological in origin, or not derived from living organisms. Click on Term to Read More organicPertaining to C-containing compounds. Organic compounds can be formed by both biological and non-biological (abiotic) processes. Click on Term to Read More compounds, such as CH4 (methane). Serpentine can be found in a few various meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More types, but is by far most prevalent in CM carbonaceous chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More. However, it has also been found in some Martian nakhlites as well. Serpentine and its group of associated minerals form at relatively low temperatures as low as ~300 K and can remain thermally stable to ~900 K (depending on conditions). The presence of serpentine is one indicator that a meteorite has undergone alteration in the presence of water. The presence of carbonates is another1.
The three mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More polymorphs of serpentine are antigorite and lizardite, which occur as massive aggregations, and chrysotile, which is fibrous. Chrysotile is one of the main forms of asbestos. A misfit between the octahedral and tetrahedral sheets causes bending, which is distributed into corrugations in antigorite and causes chrysotile to curl into cylindrical (often hollow) tubes. The idealized formula of these three polymorphs is Mg3(Si2O5)(OH)4, although in nature some of the magnesium ions are typically replaced by iron or other cations. Most serpentine in CM chondriteClass of carbonaceous chondrites named after the Mighei meteorite that fell in Ukraine in 1889. They represent samples of incompletely serpentinized primitive asteroids and have experience extremely complex histories. CM meteorites are generally petrologic level type 2 though a few examples of CM1 and CM1/2 also exist. Compared to CI Click on Term to Read More matrices appear to be Fe-bearing chrysotile and current research suggests that chrysotile growth is favored in fluid-filled voids. Even within the same CM meteorite, the various regions of serpentine can exhibit a very wide range Mg-Fe substitution.
The composition of serpentine is very uniform. The most important substitutionReplacement of one ion or ionic group for another in the same structural site in a mineral yielding a solid solution. Most substitution in minerals is of cations which are smaller and essentially sit in a lattice of oxygen anions. Anionic substitution does occur in halides. Substitutions are classified based is Fe2+ ↔ Mg2+ ↔ Ni2+ with a much less important substitution of Al3+ ↔ Si4+. Chrysotile shows least chemical deviation from ideal composition.

When most of the Mg is replaced by Fe, the resulting mineral is called cronstedtite or sometimes referred to as Fe-rich serpentine. Cronstedtite is another major constituent of CM chondrites. Note that cronstedtite is not a pure endmember but always contains some Mg in solid solutionCompositional variation resulting from the substitution of one ion or ionic compound for another ion or ionic compound in an isostructural material. This results in a mineral structure with specific atomic sites occupied by two or more ions or ionic groups in variable proportions. Solid solutions can be complete (with.
Additional reading:
- Serpentinite:What, Why, Where?
- Ferric saponite and serpentine in the nakhlite martian meteorites
- Lizardite versus antigorite serpentinite: Magnetite, hydrogen, and life(?)
- Serpentinization and the Formation of H2 and CH4 on Celestial Bodies (Planets, Moons, Comets)






