CK4

Found January 21, 2000
18° 38.6′ N., 54° 25.8′ E. A single black, 184 g, fusion-crusted stone was found in Oman. This Karoonda-type carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More has a dark-gray, porous, friable, fine-grained matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More (65 vol%), containing chondrulesRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More (up to 1 mm) and plagioclase-rich objects, but lacking FeNi-metal. The chondrules contain primary glass and have well-defined boundaries, indicative of a low degree of thermal metamorphism. Likewise, the plagioclase-rich objects have well-defined boundaries and have a wide compositional range of feldsparAn alumino-silicate mineral containing a solid solution of calcium, sodium and potassium. Over half the Earth’s crust is composed of feldspars and due to their abundance, feldspars are used in the classification of igneous rocks. A more complete explanation can be found on the feldspar group page. Click on Term to Read More, further indication of a low petrologic typeMeasure of the degree of aqueous alteration (Types 1 and 2) and thermal metamorphism (Types 3-6) experienced by a chondritic meteorite. Type 3 chondrites are further subdivided into 3.0 through 3.9 subtypes.. Other properties inherent in this meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More suggest that it experienced oxidizingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions during nebular condensation. The very low S content and lack of troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More is consistent with the removal of S as an oxide during nebular processes.
The CK
chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, although recognized as closely related to the
CV chondritesMeteorite class named after the Vigarano meteorite that fell in Italy in 1910. They have abundant large, well-defined rimless (?) chondrules of magnesium-rich olivine (~0.7 mm diameter; 40-65 vol. %), often surrounded by iron sulfide. They also contain 7-20 vol. % CAIs. The often dark-gray matrix is dominated by Fe-rich Click on Term to Read More in bulk O-isotopic composition, mineralogy, and
petrologyScience dealing with the origin, history, occurrence, chemical composition, structure and classification of rocks. Click on Term to Read More, were designated as a separate group in 1990 based on their lower abundances of refractory lithophiles and
CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More, in addition to the low abundance of coarse-grained igneous rims around chondrules in the CK group compared to the CV group; this was considered to be a result of a higher degree of
metamorphicRocks that have recrystallized in a solid state due to changes in temperature, pressure, and chemical environment. Click on Term to Read More recrystallization in CK chondrites. Igneous rims were subsequently identified in ~60% of chondrules observed in CK3–4 meteorites by Chaumard and Devouard (2016). The group was named for the observed
fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More in Karoonda, Australia, which was classified as a CK4.
Historically, CK chondrites constituted a heterogeneous group of meteorites that had refractory lithophile abundances intermediate between those of the CO and CV groups, and that had a significant abundance of altered
refractory inclusionsInclusions found predominantly in carbonaceous chondrites and are rich in refractory elements particularly calcium, aluminum and titanium that in various combinations form minerals such as spinel, melilite, perovskite and hibonite. There are two types of refractory inclusion: • Ca Al-rich inclusions (CAIs) • Amoeboid olivine aggregates (AOAs) Refractory inclusions were Click on Term to Read More. The CK group members have O-isotopic compositions that overlap those of the CV group, and the two groups also overlap in both textural and compositional variation. CK chondrites generally have low
chondruleRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More to matrix ratios, with matrix composing ~50–70 vol%, which is higher than most CV-group members. However, Chaumard and Devouard (2016) reported a large range in chondrule abundances among CK chondrites in their study, which they attributed to re-equilibration during metamorphism. In a comparison of chondrule sizes between the CK and CV meteorites, both groups show similar ranges. While the CV group has low petrologic grades, members of the CK group have been equilibrated to higher petrologic grades of ~3.5 and above. It was first proposed by Greenwood
et al. (2009) that these two meteorite groups might represent a single, thermally stratified ‘onion-shell-like’
parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More, and subsequent studies by many investigators have provided valuable evidence towards the resolution of this question.
As with the
oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More CV group, the CK group has a high
oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More state which has resulted in a very low content of FeNi-metal and a correspondingly high content of
magnetiteFe oxide, Fe2+Fe3+2O4, containing oxidized iron (Fe3+) found in the matrix of carbonaceous chondrites and as diagnostic component in CK chondrites. In CK chondrites, magnetite is typically chromian, containing several wt. % Cr2O3. Click on Term to Read More and sulfides. The dispersion of these sub-µm- to µm-sized magnetite and sulfide (
pentlanditeFe-Ni sulfide, (Fe,Ni)9S8, that is often associated with troilite, and found in the matrix and chondrules of CO, CV, CK and CR chondrites. The color is yellow-bronze with light bronze-brown streak and metallic luster. It typically forms during cooling of magmatic sulfide melts during the evolution of parent silicate melt. The Click on Term to Read More) grains within vesicles of like size has caused pronounced
silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the darkening in all metamorphic grades. The magnetite grains present in both CK and CV group members have been metasomatically altered by fluids having similar O-isotopic compositions (Davidson
et al., 2013). Other experiments have demonstrated that sub-µm- to µm-sized vesicles and micron-sized inclusions are produced during shock-melting of fine-grained matrix olivines (Hashiguchi
et al., 2008). These shock events occurred under conditions of low shock pressures (<25 GPa) and high temperatures (>600°C).
The typical features of the CK group listed above were re-evaluated by Greenwood
et al. (2003), and it was further established that the predominantly equilibrated members of the CK group were consistent with metamorphic progression of the CV group. It was suggested that the few unequilibrated CK members, such as Dhofar 015, do not exhibit the typical features of CK chondrites, but more closely resemble the oxidized CV3 chondrites.
A petrologic study was conducted by Chaumard
et al. (2009, 2011) comparing the CK chondrites to the oxidized subgroup of CV chondrites. They found that matrix, chondrule, and
CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More abundances in CK chondrites are similar to those features in some oxidized CV members. Moreover, dark inclusions commonly present in the CV group are also abundant in the CK group. In their studies they determined that CK chondrites have an
olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More chemistry that is correlated with the textural equilibration of the matrix grains. Moreover, Greenwood
et al. (2010) found that both the CK and CV chondrites contain magnetites which are compositionally similar, and that major and trace elements overlap between the groups. In addition, in their studies of discrimination diagrams, Isa
et al. (2012), found that no significant nebular-based distinctions exist between the CV and CK groups. Taking these findings into consideration, these investigators suggest that the CK group may not represent a separate parent body, but instead, consider it more likely that these meteorites constitute a metamorphic continuum derived from the more unequilibrated CV subgroup members.
It was previously recognized that the structural order of insoluble polyaromatic
organicPertaining to C-containing compounds. Organic compounds can be formed by both biological and non-biological (abiotic) processes. Click on Term to Read More matter is irreversibly transformed by thermal metamorphism (carbonization through graphitization) to a commensurate degree across meteorite chemical classes (Bonal
et al., 2005; 2007). A positive correlation exists between the particular maturation grade of organic matter and the peak metamorphic temperature of the meteorite, and the latter is directly associated with the petrologic type. In their Raman spectrographic study of maturation grade
vs. petrologic type for select CV and CK chondrites, Chaumard
et al. (2013) found that the transition from carbonization to graphitization in these chondrites occurs at the petrologic type for Allende (>3.6; Raman method). From their data, they concluded that the CV and CK chondrites in their study constitute a metamorphic sequence increasing as follows: Allende (carbonization) ⇒ NWA 779 (graphitization) ⇒ Tanezrouft 057 (graphitization) ⇒ NWA 2900 (graphitization) ⇒ NWA 1559 (graphitization) In an in-depth geochemical, mineralogical, and isotopic study of the characteristics of the CK and CV groups, Greenwood
et al. (2009) provided detailed evidence for such a common parent body scenario. They revealed that both groups show similar CRE age clusters of ~9 and ~29 m.y., and the team suggested that a classification revision be adopted in which the CK group is considered a part of the oxidized CV subgrouping and designated CV3
oxK.
Runyon and Dunn (2011) investigated Cr
2O
3 vs. MgO, and TiO
2 and NiO in magnetite and olivine, and arrived at a different conclusion from that espoused by Greenwood
et al. for a common CV-CK parent body. Their results do not support unambiguously a metamorphic sequence progressing from oxidized CV to unequilibrated and equilibrated CK meteorites. In addition, a study of
volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More abundances by Isa
et al. (2011) demonstrated no consistency with a scenario of increasing metamorphism from the oxidized CV to the unequilibrated and equilibrated CK meteorites. Furthermore, in their study of metamorphosed clasts in CV chondrites, Jogo
et al. (2011) determined that CK chondrites have higher NiO contents, have
plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More that exhibits a unique An distribution, and contain abundant magnetite. In a similar way, studies of CV–CK group relationships by Davidson
et al. (2012) led to the identification of several parameters which are inconsistent with a common CV–CK origin, including differences in chondrule Fa content and Fe/Mn ratios, and differences in FeO and Cr
2O
3 contents in opaque phases. Another study of consequence involving elemental analyses in CK chondrites was conducted by Ebihara
et al. (2012). They determined that a positive correlation exists between
REEOften abbreviated as “REE”, these 16 elements include (preceded by their atomic numbers): 21 scandium (Sc), 39 Yttrium (Y) and the 14 elements that comprise the lanthanides excluding 61 Promethium, an extremely rare and radioactive element. These elements show closely related geochemical behaviors associated with their filled 4f atomic orbital. Click on Term to Read More abundance and petrologic type, and also that REE abundances in low petrologic type CK chondrites was unlike abundances in Allende. Therefore, a scenario reflecting separate parent bodies for the CV and CK chondrites was deemed most consistent with the data. They suggest that the CK parent body was relatively small in size and had a typical onion-shell structure, with the highest metamorphic grade and the high-temperature phases residing nearest the center.
In their analyses of CV and CK chondrites spanning the entire petrographic range, Wasson
et al. (2013) demonstrated that the lack of CAIs and igneous rims, as well as the observed elemental fractionations were consistent with a more extensive metamorphic history, including impact-generated crushing, metasomatic oxidation, volatile loss, and recrystallization; Kereszturi
et al. (2015) observed that melting events also played a role in the destruction of chondrules and homogenation of the CK texture. In their in-depth study of CK chondrites, Chaumard and Devouard (2016) found a large range of peak metamorphic temperatures and a lack of correlation with petrologic types, inconsistent with a thermally stratified ‘onion-shell’ structure. They determined that CV and CK chondrites were heated at different temperatures for various durations ranging from tens of years to tens of thousand years, and argue that the heating is most consistent with solar radiative heating over a relatively long-term for an object having a close perihelion (
e.g., <0.1
AUThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More), rather than heating by radiogenic decay or impact shock.
Although Wasson
et al. (2013) believe the oxidized subgroups of the CV chondrites were originally derived from material related to the
reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More subgroup, the exact oxidation pathway is unknown. Therefore they propose a classification scheme in accord with that of Greenwood
et al. (2009) in which unequilibrated CK chondrites should be termed CV3
oxK, while the equilibrated meteorites should be designated CV4–6.
Since the proposal to combine the CV and CK groups into one metamorphic continuum was introduced, researchers have applied to the CK3 meteorites many of the same metamorphic indicators previously used to resolve the degree of metamorphism among type 3 ordinary chondrites (Dunn, 2013; Bruck and Dunn, 2014; Dunn and Gross, 2015). Among these metamorphic indicators are 1) Cr
2O
3 content in olivine from type-II chondrules, 2) percent mean deviation (PMD) of Fa in olivine from type-II chondrules, 3) NiO in olivine from type-II chondrules, and 4) average Cr
2O
3 and NiO content in magnetite. From their results, they concluded that the CK3 chondrites in their study constitute a metamorphic sequence increasing as follows:
NWA 5343—unspecified
NWA 1559—3.6 or 3.7
NWA 2043—unspecified
DaG 431—3.8
Dhofar 015—likely 3.9
Continuing the effort to better resolve the degree of metamorphism among the CK chondrites, and to determine whether or not a genetic relationship exists between the CV and CK groups, Dunn
et al. (2016) analyzed the magnetite composition in seven unequilibrated CK meteorites and in one that is equilibrated. Utilizing coupled diagrams which compare magnetite oxide abundances among CV and CK chondrites (
e.g., MgO
vs. Cr
2O
3, TiO
2, NiO, and Al
2O
3), they established geochemical and mineralogical evidence (
e.g.,
oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More fugacities, peak temperatures, magnetite compositions), as well as petrographic evidence (
e.g., chondrule size and abundance), which is most consistent with separate CV and CK parent bodies. For example, the magnetite compositions of CV chondrites should be more representative of those among the unequilibrated rather than the equilibrated CK chondrites, given a metamorphic sequence based on increasing oxidation as follows: CV3
red ⇒ CV3
ox ⇒ CK3 ⇒ CK4–6. However, in the coupled diagrams presented by Dunn
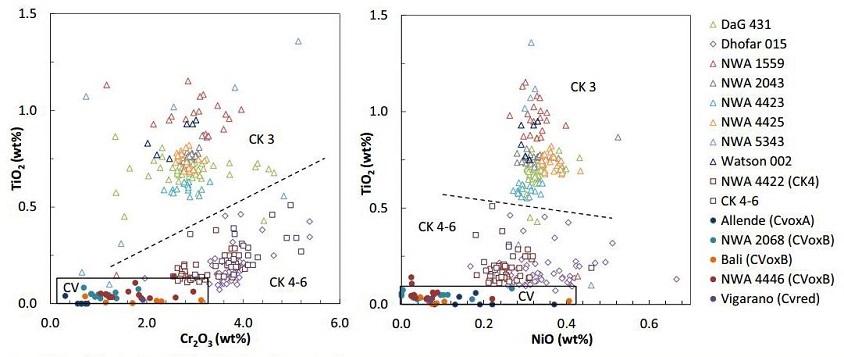
et al. (2016) this expectation is not realized (see sample diagram below). Magnetite Composition Among CV and CK Chondrites (A)

CK3 chondrites are open triangles, CK4–6 chondrites are open squares, Dhofar 015 is an open
diamondOne of the naturally occurring forms of carbon found in meteorites. Each C atom is bonded through covalent sp3 hydrid orbitals to four others. The strength of the C-C bonds makes diamond the hardest naturally occurring substance (according to the Mohs scale) in terms of resistance to scratching. There are Click on Term to Read More, and CV chondrites are solid circles.
Diagram credit: Dunn
et al.,
MAPS vol. 51, #9, p. 1711 (2016)
‘Magnetite in the unequilibrated CK chondrites: Implications for metamorphism and new insights into the relationship between the CV and CK chondrites’
(http://dx.doi.org/10.1111/maps.12691) The results of this new study based on individual
mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More chemistries are contrary to those of previous studies involving bulk compositional analyses, likely due to the heterogeneous nature of these meteorite groups (Dunn
et al., 2016). Resolution between the CV and
CK chondriteClass of carbonaceous chondrite named for the Karoonda meteorite that fell in Australia in 1930. They are more oxidized than all other carbonaceous chondrites and genetically distinct from CV chondrites. CK chondrites appear dark-gray or black due to a high percentage of Cr-rich magnetite dispersed in a matrix of dark Click on Term to Read More groups is apparent utilizing magnetite compositional diagrams (see diagrams below). A comparitive analysis was conducted by Dunn
et al. (2018) between the most unequilibrated CK
chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More known, NWA 5343 (3.6/3.7), and its possible CV-type precursor represented by the reduced CV3.3 Vigarano. They found that both the
porosityThe volume percentage of a rock that consists of void space. Vesicular porosity is a type of porosity resulting from the presence of vesicles, or gas bubbles, in igneous rock such as the pumice presented here. Vesicular porosity is very rare in meteorites and is often associated with slag, one Click on Term to Read More and the texture of matrix olivine in NWA 5343 were inconsistent with metamorphism of Vigarano-like material. Dhofar 015 is clearly distinguished from the group of unequilibrated CK chondrites; although Dhofar 015 has chondrule textures, matrix textures, and a feldspar compositional range consistent with petrologic type CK3.9 (Ivanova
et al., 2000), its
fayalitePure* iron end-member (Fe2SiO4) of the olivine solid solution series and an important mineral in meteorites. When iron (Fe) is completely substituted by magnesium, it yields the the pure Mg-olivine end-member, forsterite (Mg2SiO4). The various Fe and Mg substitutions between these two end-members are described based on their forsteritic (Fo) Click on Term to Read More value (Fa
32) and its
magnetite composition places it among the group of equilibrated CK chondrites. Therefore, Dunn
et al. (2016) contend that Dhofar 015 should be classified as an equilibrated CK4. Magnetite Composition Among CV and CK Chondrites (B)
CK3 chondrites are open triangles, CK4–6 chondrites are open squares, Dhofar 015 is an open diamond, and CV chondrites are solid circles.
Diagrams credit: Dunn
et al.,
MAPS vol. 51, #9, pp. 1712–1713 (2016)
‘Magnetite in the unequilibrated CK chondrites: Implications for metamorphism and new insights into the relationship between the CV and CK chondrites’
(http://dx.doi.org/10.1111/maps.12691) Previous studies (
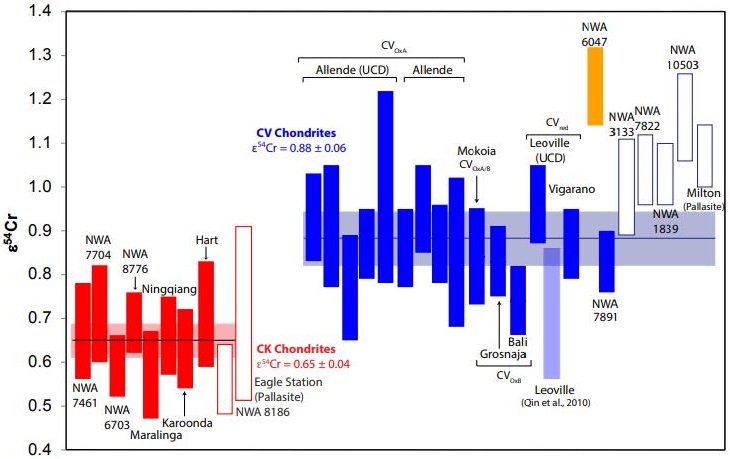
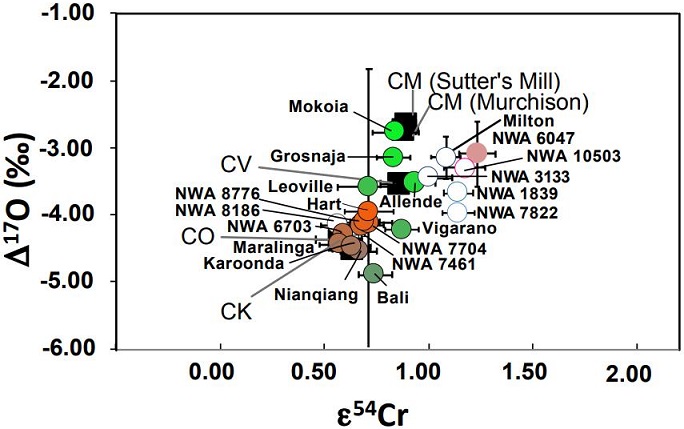
e.g., Sanborn
et al., 2014) have established that a coupled Δ
17O
vs. ε
54Cr diagram is one of the best diagnostic tools for determining genetic relationships between meteorites. New Cr-isotopic analyses were conducted by Yin
et al. (2017, 2019) for a broad sampling of CK and CV chondrites. When combined with previous analyses, it was determined that CK chondrites have an average ε
54Cr value of +0.65 (±0.04), while the CV chondrites have an average value of +0.88 (±0.06). The results are consistent with an origin from two distinct parent bodies (see diagrams below, and also the
NWA 6047 page). Cr
IsotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More Weighted Average For CV and CK Chondrites
 click on photo for a magnified view
click on photo for a magnified view
O–Cr Diagram For CV and CK Chondrites
CK: orange shades; CV: green shades; Achondrites: open
 click on photo for a magnified view
click on photo for a magnified view
Diagrams credit: Yin and Sanborn
et al., 50th LPSC,
#3023 (2019)
Dhofar 015 is a relatively fresh meteorite with a weathering grade of W1 on the Wlotzka (1993) scale, and a
shock stageA petrographic assessment, using features observed in minerals grains, of the degree to which a meteorite has undergone shock metamorphism. The highest stage observed in 25% of the indicator grains is used to determine the stage. Also called "shock level". Click on Term to Read More of S3. Meteorites constituting the equilibrated CV group have an average porosity of 14%, virtually the same as that measured for the unequilibrated CV group (ave. 14.6%; Macke
et al., 2011). The photo above shows the interior side of a 0.47 g specimen of Dhofar 015 while that below shows the fusion-crusted side.













