Iron, IIE, silicated octahedriteMost Common type of iron meteorite, composed mainly of taenite and kamacite and named for the octahedral (eight-sided) shape of the kamacite crystals. When sliced, polished and etched with an acid such as nitric acid, they display a characteristic Widmanstätten pattern. Spaces between larger kamacite and taenite plates are often Click on Term to Read More
(HH chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More related)
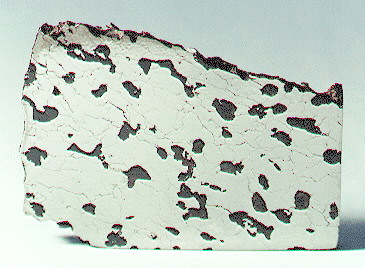
Found 1992
27° 50′ S., 150° 20′ E. A mass of about 265 kg was found by Mr. Frank Timms on open shrub farmland near Miles, in Queensland, Australia. About 100 kg was exported to the USA by M. Killgore and submitted for analysis. Miles has been classified as a group IIE iron belonging to the ‘fractionated IIE’ grouping, as distinct from the ‘normal IIE’, ‘IIE-An’, and ‘ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More iron’ categories distinguished by Wasson and Wang (1986). Researchers divided the eight known (at the time) silicate-bearing IIE irons into five groups based on silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the inclusionFragment of foreign (xeno-) material enclosed within the primary matrix of a rock or meteorite. Click on Term to Read More types: 1) chondritic clasts (Netschaëvo), 2) partially melted but undifferentiated clasts (Techado), 3) completely melted clasts with a loss of metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More and sulfide (Watson 001), 4) plagioclase–orthopyroxene–clinopyroxene basaltic partial melts (Miles and Weekeroo Station), and 5) plagioclase–clinopyroxene partial melts (Colomera, Kodaikanal, and Elga) et al., 1998). Other researchers (e.g., Ruzicka, 2014) have followed a more simplified classification systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with for the ten silicate-bearing IIE irons known at the time, recognizing only two subgroups—unfractionated (Netschaëvo, Techado, and Watson 001) and fractionated (Miles, Weekeroo Station, Colomera, Kodaikanal, Elga, Tarahumara, and NWA 5608). As a result of their classification of the silicated IIE Mont Dieu, Van Roosbroek et al. (2015) suggest that a five-stage division from most primitive to most differentiated is most useful as follows: 1) Mont Dieu and Netschaëvo; 2) Techado; 3) Watson; 4) Miles and Weekeroo Station; 5) Kodaikanal, Colomera, and Elga. On the other hand, an investigation of silicated IIE irons by McDermott et al. (2015) led them to propose a classification scheme that comprises only four categories, from most primitive to most differentiated as follows: primitive chondritic (Netschaëvo, Mont Dieu, Garhi Yasin, Techado) → evolved chondritic (Watson 001) → differentiated with high opx (>10 vol%; Weekeroo Station, Miles, Tarahumara) → differentiated with low opx (<10 vol%; Kodaikanal, Colomera, and Elga).
Miles contains 10–20 vol% coarse-grained, globular silicate inclusions which generally form an interconnected network. They have been described as feldspathic orthopyroxenitic or pyroxene-enriched
basaltBasalt is the most common extrusive igneous rock on the terrestrial planets. For example, more than 90% of all volcanic rock on Earth is basalt. The term basalt is applied to most low viscosity dark silicate lavas, regardless of composition. Basalt is a mafic, extrusive and fine grained igneous rock Click on Term to Read More/gabbro. The major silicate phases include
plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More feldsparAn alumino-silicate mineral containing a solid solution of calcium, sodium and potassium. Over half the Earth’s crust is composed of feldspars and due to their abundance, feldspars are used in the classification of igneous rocks. A more complete explanation can be found on the feldspar group page. Click on Term to Read More, clinopyroxene (
augiteHigh-Ca clinopyroxene, (Ca,Mg,Fe)SiO3, that occurs in many igneous rocks, particularly those of basaltic composition. In order to be considered augite, the clinopyroxene must contain 20 to 45 mol % of calcium (Wo20 - 45). An important and unique Martian meteorite is NWA 8159, that has been classified as an augite basalt. Click on Term to Read More), and
orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More in a ratio of 2:1:1. In addition, albitic glass, whitlockite, chlorapatite, merrillite,
schreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More, and
chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More occur, along with accessory
tridymiteSilica group mineral in which the tetrahedra occur in sheets. Tetrahedra alternately point up or down to share oxygen with tetrahedra of other sheets, forming six-sided rings perpendicular the sheets. Tridymite has a fairly open structure and accommodates Na+, K+ and Ca2+; charge balance is achieved by Al3+ ↔ Si4+., K-feldspar, antiperthite, and rare FeS. The low abundance of sulfide has been attributed to evaporative loss of S in an FeS melt near the surface of the
parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More (Ikeda and Prinz, 1996).
Studies based on W-isotopic systematics by Schulza
et al. (2012) indicate that IIE irons experienced multiple metal segregation events at ~3, ~13, and ~28 m.y. after
Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. formation. The two later events occurred after radiogenic heating had diminished and are best attributed to impact heating. They also argue that the Sm-isotopic data are most consistent with the precursor material of the Miles
meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More not being exposed on the parent body surface.
The inclusions were likely derived from both slowly cooled
equilibriumTerm used to describe physical or chemical stasis. Physical equilibrium may be divided into two types: static and dynamic. Static equilibrium occurs when the components of forces and torques acting in one direction are balanced by components of forces and torques acting in the opposite direction. A system in static Click on Term to Read More processes that occurred between
cumulateIgneous rock composed of crystals that have grown and accumulated (often by gravitational settling) in a cooling magma chamber. Click on Term to Read More and melt phases lasting ~20 t.y., and from a more rapidly cooled, late-stage (following ~98%
crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More)
fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More phase of much shorter duration (Ruzicka and Hutson, 2009). This latter rapid cooling phase is consistent with a catastrophic collision that disrupted the moderately melted and partially differentiated (structurally zoned) planetesimal, which retained a chondritic
crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More. This was followed by the re-accretion of one or more smaller second-generation asteroids composed predominantly of metallic melt with some silicate mush; it is these heterogeneous second-generation asteroids from which the silicated IIE meteorites such as Miles likely originated (Ruzicka, 2014).
Another type of inclusion has a
cryptocrystallineCrypto meaning "hidden" refers to a rock texture in which individual crystals are too small to be distinguished even using a standard petrographic microscope. Crystals are typically less than a few μm in size - any smaller and the texture would be considered amorphous. Among sedimentary terrestrial rocks, chert and Click on Term to Read More texture and is present in lower abundance compared to the gabbroic inclusions. It is thought to have formed by late-stage impact-shock of albite-rich inclusions which were cooled quickly. In addition,
orthopyroxeniteA rock composed primarily of orthopyroxene. Non-terrestrial orthopyoxenites include diogenites and a single martian meteorite, ALH 84001, that was found in the Allan Hills region of Antarctica in 1984. ALH 84001 is a cumulate rock consisting of 97% coarse-grained, Mg-rich orthopyroxene, with small amounts of plagioclase, chromite, and carbonate. It Click on Term to Read More inclusions and a rhyolitic assemblage have also been identified (Ruzicka and Hutson, 2009). While there is large variation in bulk compositions among the inclusions, they have similar major- and trace-element compositions consistent with derivation from a common precursor. In Miles and other IIE irons with differentiated silicates, a wide variety of shock features are present corresponding to
shock stageA petrographic assessment, using features observed in minerals grains, of the degree to which a meteorite has undergone shock metamorphism. The highest stage observed in 25% of the indicator grains is used to determine the stage. Also called "shock level". Click on Term to Read More S4 and postshock heating up to ~300°C.
The small, globular silicates present in Miles (as well as Colomera, Weekeroo Station, Kodaikanal, and Elga) likely formed in a hot, highly differentiating environment, such as within a multi-km-deep
regolithMixture of unconsolidated rocky fragments, soil, dust and other fine granular particles blanketing the surface of a body lacking an atmosphere. Regolith is the product of "gardening" by repeated meteorite impacts, and thermal processes (such as repeated heating and cooling cycles). Click on Term to Read More. At peak temperatures of 1250°C, the proportion of liquid metal exceeded that of solid metal by as much as a 2:1 ratio. Other IIE irons contain melted but undifferentiated (primitive) silicates (Watson, Techado), or even unmelted, chondrule-bearing silicates (Netschaëvo, Mont Dieu), and these groups provide evidence for mechanical disruption and mixing of very diverse lithologies on an H-like chondritic parent body. Both the wide range of cooling rates and the varying degrees of
differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More exhibited by the silicates suggest a near-surface cooling environment having a large temperature gradient, or alternatively, multiple impact events of varying scales. The discovery of quenched impact-melt clasts in Netschaëvo led Roosbroek
et al. (2016) to conclude that this IIE silicated iron is actually a
brecciaWork in Progress ... A rock that is a mechanical mixture of different minerals and/or rock fragments (clasts). A breccia may also be distinguished by the origin of its clasts: (monomict breccia: monogenetic or monolithologic, and polymict breccia: polygenetic or polylithologic). The proportions of these fragments within the unbrecciated material Click on Term to Read More. They recognized two different metal/silicate lithologies which experienced distinct petrogenetic histories: i) a metamorphosed (type 6–7), chondrule-bearing lithology formed through early indigenous radiogenic heating, and ii) an impact-melt rock (IMR) lithology most likely derived from the former during the late-stage impact event dated at ~3.7 b.y. ago.
Miles contains silicates that formed at significant depth and which experienced a high degree of fractional crystallization at higher temperatures and slower cooling conditions. The presence of undevitrified albitic glass inclusions in some IIE members implies a more rapid cooling (within a few days) from a melt at lower temperatures. A rapid cooling history is also necessary to explain the lack of segregation of low-density silicates from the high-density FeNi-metal host, as well as the small size of the
taeniteLess common than kamacite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Taenite, γ-(Fe,Ni), has 27-65 wt% Ni, and forms small crystals that appear as highly reflecting thin ribbons on the etched surface of a meteorite; the name derives from the Greek word for "ribbon." Click on Term to Read More crystals (Wasson, 1972). Based on a thorough investigation into the petrogenesis of
Sombrerete, a silicated iron tentatively grouped within the IAB iron complex, Ruzicka
et al. (2006) argued that the evolved members of the IIE irons, and probably some silicated irons from other groups, experienced a two-stage formation history similar to that of Sombrerete.
An alternative mechanism for the formation of the inclusions and metal host in IIE irons has been proposed by Kurat
et al. (2007). They believe that the many contradictory features observed in these irons, such as the discordance in age between inclusions and metal, the chemical and isotopic disequilibria among various components, the Eu and Yb abundance anomalies, the presence of glasses, and the ability for components with such highly contrasting
densitiesMass of an object divided by its volume. Density is a characteristic property of a substance (rock vs. ice, e.g.). Some substances (like gases) are easily compressible and have different densities depending on how much pressure is exerted upon them. The Sun is composed of compressible gases and is much Click on Term to Read More to form a pore-free, uniform assemblage, reflect nebular
fractionationConcentration or separation of one mineral, element, or isotope from an initially homogeneous system. Fractionation can occur as a mass-dependent or mass-independent process. Click on Term to Read More and metasomatic processes rather than formation through an impact-shock scenario. These investigators argued that the pyroxenes and apatite that are embedded in the glass inclusions obviously crystallized from the same precursor liquid phase, but that their compositions are now far out of equilibrium with the present glassy
mesostasisLast material to crystallize/solidify from a melt. Mesostasis can be found in both chondrules, in the matrix around chondrules, and in achondrites as interstitial fine-grained material such as plagioclase, and/or as glass between crystalline minerals. Click on Term to Read More, which is especially evident as a high depletion of
REEOften abbreviated as “REE”, these 16 elements include (preceded by their atomic numbers): 21 scandium (Sc), 39 Yttrium (Y) and the 14 elements that comprise the lanthanides excluding 61 Promethium, an extremely rare and radioactive element. These elements show closely related geochemical behaviors associated with their filled 4f atomic orbital. Click on Term to Read More in the glass. They propose that the silicate inclusions were initially formed from a high-temperature, refractory-element-rich liquid that condensed from a non-fractionated
nebulaAn immense interstellar, diffuse cloud of gas and dust from which a central star and surrounding planets and planetesimals condense and accrete. The properties of nebulae vary enormously and depend on their composition as well as the environment in which they are situated. Emission nebula are powered by young, massive Click on Term to Read More gas under
reducingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions, and which thereafter was quenched to glass. The occurrence of metasomatic processes in this nebular region, similar to that in which ordinary
chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More were formed, resulted in the Si-rich, alkali-rich, REE-depleted compositions we observe. The metal phase is posited to have condensed around the inclusions under low-temperature conditions, possibly from carbonyl breakdown.
The radiometric Ar–Ar age of Miles, reflecting its time of crystallization from a melt, was calculated to be ~4.41 b.y. At least five of the other IIE silicated irons share this approximate age (
e.g., Colomera, Weekeroo Station, Tarahumara, Techado, and Mont Dieu), while Watson, Kodaikanal, and Netschaëvo share younger ages of ~3.68 b.y., all of which likely experienced resetting by a common localized impact event. Cosmic-ray exposure ages for the IIE members also plot into distinct groups, with one group again comprising Watson, Kodaikanal, and Netschaëvo sharing CRE ages of ~3–15 m.y., and another group comprising the remaining IIE members having much older CRE ages, up to ~400 m.y. Based on these age distinctions, it is reasonable that Watson, Kodaikanal, and Netschaëvo could have been ejected from a unique location during a common impact event. Any CRE age differences that do exist for these three meteorites can be interpreted as being the result of shielding or late breakup events, or in the case of Netschaëvo, from forging to >1000°C after its recovery.
There have been a number of formation models presented to explain the mixing of metal and silicates observed among the spectrum of IIE meteorites:
Scenario 1
Both metal and silicate fractions underwent low degrees of
partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More (~30%) and incomplete segregation as a result of endogenous heating to peak temperatures of ~1250°C. Silicate melts underwent fractional crystallization prior to becoming trapped within small metallic melt sheets or pods (similar in size to IAB iron subgroups; Wasson and Scott, 2011) within the silicate crust or upper
mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More, forming the IIE meteorites.
Scenario 2
Through the impact of an FeNi-metal object derived from the
coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More of a differentiated H chondrite-like body, both metal and silicate fractions formed in near-surface impact-melt sheets or pools on a porous chondritic planetesimal similar to the
H chondriteOrdinary chondrites with a high content of free Ni-Fe metal (15-19 vol. %) and attracted easily to a magnet. Their main minerals are olivine (Fa16-20) and the orthopyroxene bronzite (Fs14.5-18.5), earning them their older name of bronzite chondrites. Chondrules average ~0.3 mm in diameter. Comparison of the reflectance spectra of Click on Term to Read More parent body (Gaffey and Gilbert, 1998). This scenario is consistent with W-isotopic data which indicate late metal segregation events occurring ~13 and ~28 m.y. after solar system formation. This was followed by mixing in subsequent impacts and by rapid lithification to produce IIE meteorites.
Scenario 3
A hybrid of the above scenarios has been suggested in which endogenous heating of an H chondrite-like parent body produced low degrees (~7%) of partial melting followed by migration of basalt, FeNi-metal, and FeS melts, leaving olivine–
pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More residues. Varying degrees of differentiation resulted in a variety of lithologies that were then collisionally mixed within metallic melt sheets or pods. Impact heating (and heat from adjacent molten FeNi-metal) remelted plagioclase and pyroxene to produce the albitic glass. The entire composite was then rapidly cooled to produce silicated IIE meteorites. The three members of the IIE group which have young radiometric ages, Watson and Netschaëvo, likely experienced age resetting by large-scale impacts long after their initial formation. Evidence suggests Kodaikanal experienced late-stage impact melting and mixing to produce its differentiated silicates.
Scenario 4
As outlined above, Kurat
et al. (2007) propose a model by which the components of the IIE group were formed within a condensing nebula region rather than through impact-shock melting processes on accreted
planetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More. They submit that nebular metasomatic processes were the mechanism for the compositional alteration observed today.
Many models have been developed that place the origin of the IIE iron group on the H-chondrite parent body, considered to be an S-IV type asteroid such as 6 Hebe or a similar H chondrite-like parent body. A surface consisting of 40% FeNi-metal and 60% H5 chondrite would match the S-type spectrum of 6 Hebe. Asteroid 6 Hebe is located next to the 3:1 and ν
6 resonances which serve as a major source of meteorites delivered to Earth. Mo-isotopic anomalies are correlated with Ru-isotopic anomalies related to
s-process material synthesized in low-mass
AGB starsStars on the Asymptotic Giant Branch, which represents a late stage of stellar evolution that all stars with initial masses < 8 Msun go through. At this late stage of stellar evolution, gas and dust are lifted off the stellar surface by massive winds that transfer material to the interstellar Click on Term to Read More (Dauphas
et al., 2004). The ε
100Ru values for IIE irons overlap with those of ordinary chondrites, consistent with a genetic relationship with H chondrites (Fischer-Gödde, 2015). O-isotopic compositions (McDermott
et al., 2010, 2011), petrographic evidence, and some CRE ages link these two groups and suggest a model for their joint formation: A plausible scenario for the formation of Miles was proposed by Ikeda
et al. (1997) and Ebihara
et al. (1997). Large impacts onto a porous, metal-rich, H chondrite-type asteroid produced partial melting of ~25%, forming localized melt sheets and pods at the bottoms of craters. The melt consisted of both a silicate phase and an Fe–Ni–S–P phase, along with a residual phase of
olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More and orthopyroxene. Incipient crystallization of the silicate melt phase produced a crystal mush consisting primarily of phenocrysts of
pigeoniteLow-Ca clinopyroxene, (Ca,Mg,Fe)SiO3, found as a major mineral in eucrites and shergottites. In order to be considered pigeonite, the clinopyroxene must contain 5 to 20 mol % of calcium (Wo5 - 20). Chondrites of petrologic types 4 and below contain significant low-Ca clinopyroxene. During metamorphism to higher temperatures, all existing, orthopyroxene, and plagioclase, which was then mixed with the Fe–Ni–S–P melt phase to produce the gabbroic (low proportion of residual melt) and cryptocrystalline (high proportion of residual melt) inclusions within the host metal. Half of the phosphorus from the Fe–Ni–S–P melt phase was utilized in the
reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More of the silicate inclusions to form metal and phosphates, while the phosphate formation in turn utilized much of the CaO from
anorthiteRare compositional variety of plagioclase and the calcium end-member of the plagioclase feldspar mineral series with the formula CaAl2Si2O8. Anorthite is found in mafic igneous rocks such as anorthosite. Anorthite is abundant on the Moon and in lunar meteorites. However, anorthite is very rare on Earth since it weathers rapidly Click on Term to Read More in the plagioclase. During this reduction process, Cu behaved as a chalcophile
elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More and was sequestered into sulfide, while Ga was transferred into host metal in higher than normal abundances. Rapid cooling at low temperatures near the surface and near the edge of the melt pools resulted in glass formation within the inclusions, and the eventual
exsolutionSegregation, during cooling, of a homogeneous solid solution into two or more different solids. Click on Term to Read More of the remaining phosphorus to form schreibersite around silicate inclusions. Those areas which cooled more slowly underwent extensive fractional crystallization, accounting for the large variations in incompatible element abundances (Lindsay
et al., 2003).
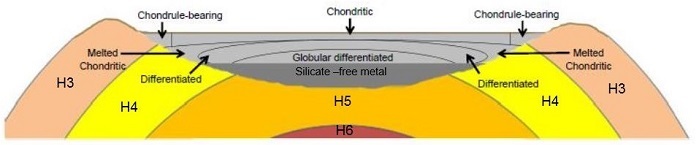
Schematic diagram of an impact melt pool origin for IIE irons
Diagram credit: McDermott
et al., 45th LPSC
#1910 (2014) Utilizing precise Hf–W chronometry in a study of nine IIE irons, Fisher-Gödde
et al. (2016) ascertained ε
182W values which attest to the occurrence of three separate metal–silicate segregation events on the parent body:
- 3.7–5.3 m.y. after CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More (Colomera, Barranca Blanca, Arlington, Mont Dieu)
- 10–15 m.y. after CAIs (Weekeroo Station, Watson, Miles, Kodaikanal)
- ~27 m.y. after CAIs (Tarahumara)
In addition, on a CRE-corrected coupled ε100Ru vs. ε92Mo diagram, these nine IIE irons plot with ordinary chondrites indicating a probable genetic relationship; i.e., IIE irons formed through impact-generated melting on an ordinary chondriteWork in Progress Ordinary chondrites (OCs) are the largest meteorite clan, comprising approximately 87% of the global collection and 78% of all falls (Meteoritical Society database 2018)1. Meteorites & the Early Solar System: page 581 section 6.1 OC of type 5 or 6 with an apparent shock stage of S1, Click on Term to Read More parent body during several impact events over an extended period of time. In addition, the IVA irons plot with the IIE irons and ordinary chondrites, and all of these groups likely originated in a similar reservoir (see diagram below). 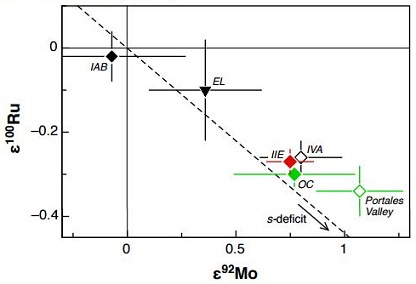
Diagram credit: Fisher-Gödde et al., 47th LPSC, #2704 (2016) In contrast to the ‘nonmagmatic’ IAB complex irons, the IIE precursor material contained a lower abundance of volatiles such as S and C, and consequently the melting temperatures were higher, resulting in silicates with nonchondritic compositions (Wasson and Wang, 1986). The metal–sulfide-rich H6 meteorite Y-791093 contains both chondritic and metal–sulfide components which are texturally, mineralogically, and compositionally similar to members of the H-chondrite group. It might be transitional between the H chondrites like Rose City and the primitive IIE irons with silicate inclusions like Netshaëvo (Ikeda et al., 1997). Similar to Miles, Y-791093 lacks a Thomson (Widmanstätten) structure and probably formed at a shallow depth rather than in a core.
Teplyakova
et al. (2012) conducted studies on the differentiation of the IIE iron group. Based on comparisons of siderophile elements between IIE metal and H-chondrites, they determined that that all of the IIE irons are consistent with formation as solid metal which had precipitated from a metallic liquid of H-chondrite composition. It was determined that even metal from Miles, which has the most fractionated siderophile pattern of the group, precipitated from the parental liquid after ~70% crystallization of solid metal. These findings are contrary to a scenario involving quenching within cooler silicates following an impact, but instead suggest crystallization from a melt within a reducing, endogenously-heated environment.
Utilizing new analytical techniques for measuring O-isotopic data, McDermott
et al. (2011) found nearly identical mean Δ
17O values for IIE irons and H chondrites. However, despite this finding and the data presented above, several factors suggest a different conclusion for the origin of the IIE group. Certain undifferentiated silicates in some IIE members contain FeO abundances that are below the range for H chondrites. Moreover, IIE O-isotopic compositions may only appear the same as those in H chondrites due to a lack of precision in the estimates and to the wide range of values. The O-isotopic values collected by Clayton
et al. (1991) and Clayton and Mayeda (1996) were 0.14‰ lower for IIE irons than for H chondrites. In addition, Wasson and Scott (2011) documented significant differences between the metal in the Portales Valley H chondrite and in IIE irons on Co–
AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More and Ga–Au plots. Moreover, olivine and chromite grains in the IIE irons which contain relict
chondrulesRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More (
e.g., Netschaëvo, Mont Dieu) have Fa and Fs ranges as well as O-isotopic compositions distinct from H chondrites (Schrader
et al., 2017). Furthermore, the CRE ages of the majority of the IIEs are much older than any H chondrite. Therefore, it is reasonable to conclude that the two groups formed on similar but separate parent bodies. The IIE meteorites may originate from an H chondrite-like parent body that experienced more extensive differentiation, was more
reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More, and which had higher abundances of
maficOne of the two broad categories of silicate minerals, the other being felsic, based on its magnesium (Mg) and/or iron (Fe) content. Mafic indicates silicate minerals that are predominantly comprised of Mg and/or Fe.The term is derived from those major constituents: Magnesium + Ferrum (Latin for iron) + ic (having Click on Term to Read More silicates and metal, higher abundances of siderophile elements, and slightly different O-isotopic compositions—an ‘HH’ chondritic asteroid (Teplyakova
et al., 2012; J. T. Wason, 2017).
In an updated study of H chondrites and IIE silicated irons based on high precision O-isotopic data, McDermott
et al. (2015) suggest that H chondrites (as well as HH chondrites) might derive from multiple source objects, with some possibly sharing a common parent body with IIE silicated irons. They argue that all of the IIE irons likely formed on a common large H/HH chondritic body that was heated internally by radiogenic elements but had not experienced significant differentiation. Following a severe impact disruption event, rapid cooling ensued preventing metal/silicate unmixing in some regions, succeeded by burial from re-accreting material which initiated a period of slow cooling. Late, less severe impact events occurred which established the ~3.7 b.y. old chronometric age of Netschaëvo and others, and fragments were ejected on a trajectory to Earth.
Dey
et al. (2019) employed
17O and ε
54Cr values for several irons and their associated silicates/oxides to investigate i) if each iron and the associated phases originated on a common parent body (
i.e., an endogenous mixture of core and mantle
vs. an exogenous mixture through impact), and ii) if any genetic connection exists between the irons/pallasite and other meteorite groups (
e.g., IAB with winonaites, IIE with H chondrites, and Eagle Station pallasites with CK chondrites). Two primitive IIE irons were included in the study, Netschaëvo and Watson 001, along with the H metallic melt breccia Portales Valley. It was demonstrated on a coupled diagram (see below) that the ε
54Cr values for silicates in Portales Valley and both the silicates and the oxide phase (chromite) in Netschaëvo and Watson 001 are identical within error and plot with the H group chondrites. Other results from their study can be found on the
Caddo County and
Eagle Station pages.
17O
vs. ε
54Cr for Irons and Pallasites
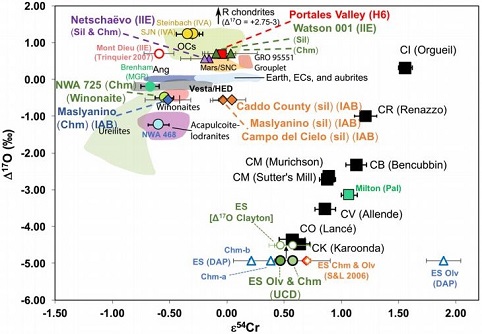 click on photo for a magnified view
click on photo for a magnified view
Diagrams credit: Dey
et al., 50th LPSC,
#2977 (2019)
Prior interest in asteroid 6 Hebe as the source of the H chondrites has lost some favor after hydrocode model data revealed inconsistencies between expected and observed CRE ages based on the scenario of direct injection into resonances. Studies by Rubin and Bottke (2009) on this subject have led them to conclude that family-forming events resulting in large
meteoroidSmall rocky or metallic object in orbit around the Sun (or another star). reservoirs having homogenous compositions, and which are located near dynamical resonances such as the Jupiter 3:1 mean motion resonance at 2.50
AU, are the most likely source of the most prevalent falls such as H chondrites and HED achondrites. See further details on the
Abbott page. The specimen of Miles shown above is a 95 g partial slice showing abundant globular silicate inclusions.










