Sombrerete
Iron, ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More
(possibly CR chondriteClass named for the Renazzo meteorite that fell in Italy in 1824, are similar to CMs in that they contain hydrous silicates, traces of water, and magnetite. The main difference is that CRs contain Ni-Fe metal and Fe sulfide that occurs in the black matrix and in the large chondrules Click on Term to Read More related)
Found 1958
23° 38′ N., 103° 40′ W. A single mass of ~10 kg was found in Sombrerete, Zacatecas, Mexico. Sombrerete was initially considered to be an anomalous iron related to the small non-magmatic IIE group, some members of which contain similar globular silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the inclusions, and it was routinely included in studies of this group. However, the silicate inclusions in Sombrerete have O-isotopic compositions that plot far away from those of IIE irons, and furthermore, the Δ17O is significantly more negative than those for typical IAB irons; this suggests an origin for the sHL subgroup on a different asteroid (Wasson, 2011). Notably, the Δ17O of Sombrerete is nearly identical to that of the silicate-bearing NWA 468, the two showing only a small difference in mass fractionationFractionation of isotopes or elements that is dependent on their masses. Click on Term to Read More on an oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More three-isotope diagram. As demonstrated by its plot on a Δ17O vs. ε54Cr diagram, NWA 468 is now strongly considered to be an anomalous, metal-rich lodraniteRare type of primitive achondrite named after the Lodran meteorite that fell in Pakistan in 1868. Initially, lodranites were grouped with the stony-iron meteorites because they contain silicates (olivine, orthopyroxene, and minor plagioclase) and Fe-Ni metal in nearly equal proportions. However, since discovery of the closely related acapulcoite group, lodranites Click on Term to Read More (Sanborn et al., 2014). It has been demonstrated that the acapulcoite–lodranite clan accreted in the non-carbonaceous reservoir, which remained separated from the carbonaceous reservoir in the early Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. due to the rapid accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More of proto-Jupiter. Contrariwise, it has been demonstrated through Mo- and Os-isotopic analyses that Sombrerete formed in the carbonaceous reservoir closely allied with group IVB irons (Worsham et al., 2017). Oxygen IsotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More Compositions of Silicate-bearing Irons
Diagram credit: A. Ruzicka, Chemie der Erde–GeochemistryStudy of the chemical composition of Earth and other planets, chemical processes and reactions that govern the composition of rocks and soils, and the cycles of matter and energy that transport Earth's chemical components in time and space. Click on Term to Read More, vol. 74, no. 1, p. 6 (Mar 2014)
‘Silicate-bearing iron meteorites and their implications for the evolution of asteroidal parent bodies’
(https://doi.org/10.1016/j.chemer.2013.10.001)

click on image for a magnified view

Diagram credit: Scott et al., The Astrophysical Journal, vol. 854, #2 (2018)
‘Isotopic Dichotomy among Meteorites and Its Bearing on the Protoplanetary Disk’
(https://doi.org/10.3847/1538-4357/aaa5a5) In a study of the IAB subgroups, employing precise Mo, W, and Os isotope data along with HSE and other literature data, Worsham et al. (2017) demonstrated on a coupled µ97Mo vs. µ189Os diagram that Sombrerete plots with the IVB magmatic irons. Notably, the ungrouped iron Chinga also plots with Sombrerete and the IVB irons, attesting to the formation of these meteorites in a common reservoir in a spatial and/or temporal aspect (see the Chinga page). Two distinct reservoirs existed in the early protoplanetary disk—carbonaceous chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More (CC) and non-carbonaceous (NC). These reservoirs were segregated by the rapid accretion of proto-Jupiter and reflect differences in the contribution (i.e., susceptibility to thermal processing) of p-, r-, and s-process isotopes inherited as dust ejectaFractured and/or molten rocky debris thrown out of a crater during a meteorite impact event, or, alternatively, material, including ash, lapilli, and bombs, erupted from a volcano. Click on Term to Read More from explosive stellar nucleosynthesis (Poole et al., 2017; Bermingham et al., 2018). It was demonstrated that the IVB irons formed is the carbonaceous chondrite reservoir (see further details in the Appendix, Part III). Since major compositional differences exist among all of these meteorites, it is likely that they represent distinct parent bodies. See diagrams below, where Sombrerete is the crossed yellow triangle and µ notation denotes deviation from terrestrial standards in parts per million. CRE-corrected Mo vs. Os for IAB Complex Irons
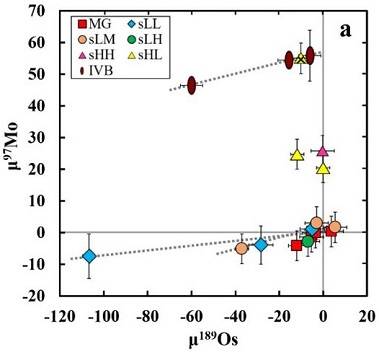 CRE-corrected Mo Isotopic Compositions of MeteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More Groups
CRE-corrected Mo Isotopic Compositions of MeteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More Groups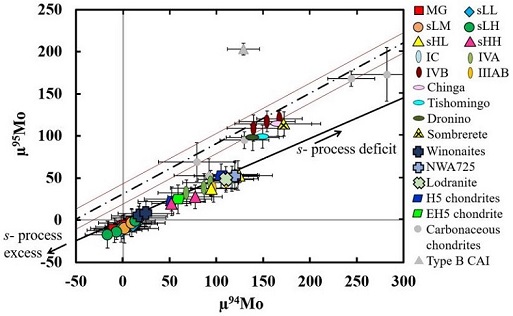
click on photo for a magnified view Diagrams credit: Worsham et al., Earth and Planetary Science Letters, vol. 467, pp. 157–166 (2017)
‘Characterizing cosmochemical materials with genetic affinities to the Earth: Genetic and chronological diversity within the IAB iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More complex’
(https://doi.org/10.1016/j.epsl.2017.02.044) With reference to the many mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More and textural similarities in their silicates, it can be argued that Sombrerete followed a similar petrogenetic path as the silicate-bearing IIE irons, and possibly other silicated irons. A taxonomic revision of the IAB–IIICD iron group was proposed by Wasson and Kallemeyn (2002), which led to the tentative inclusionFragment of foreign (xeno-) material enclosed within the primary matrix of a rock or meteorite. Click on Term to Read More of Sombrerete into the newly defined IAB complex and resolution on a Ni–AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More diagram as a member of the high-Au, low-Ni subgroup (sHL). The fact that a large concentration of sHL members has been found near Erfoud, Morocco, and that four of them might be paired (Hassi-Jekna and NWA-series members 3200, 4706, and 4710), provides evidence for the presumption that the IAB subgroups derive from separate impact-melt pools on a single unique asteroid. Although no other sHL members have undergone O-isotopic analyses, Sombrerete has a very different O-isotopic value than the other measured IAB irons (Δ17O = –1.39‰ vs. the typical ~ –0.5‰). Therefore, it may be more plausible that Sombrerete, and potentially the other sHL subgroup members, formed on a separate asteroid, perhaps related to the CR chondrite clan (Ruzicka et al., 2006; Ruzicka, 2014). In their study of the Mo isotope systematics among IAB complex irons, Dauphas et al. (2002) reported that the sHL subgroup iron Magnesia is distinct from the IAB main group. Further evidence supporting the hypothesis for separate parent bodies for Sombrerete and possibly the entire sHL subgroup was presented by Worsham and Walker (2015, 2016). They studied the Mo-isotopic compositions of representative meteorites from the IAB iron complex, and it was ascertained that Sombrerete is clearly resolved from members of the MG, sLL, and sLM subgroups of the IAB complex. They also recognized that the latter three subgroups share very similar W- and Mo-isotopic values and have Mo-isotopic values indistinguishable from that of the Earth. Notably, these subgroups represent the Earth’s closest genetic relatives. Furthermore, they found that a member of the sHH subgroup (ALHA80104) was also clearly resolved from other members of the IAB complex (MG, sLL, sLM) with respect to its Mo- and W-isotopic values and by its older metal–silicate segregation age as determined by Hf–W systematics, and therefore it was concluded that both the sHL and sHH subgroups might derive from distinct parent bodies located in separate nebular regions from other members of the IAB complex irons. Therefore, because the Δ17O value, HSE data, and Mo-isotopic composition determined for Sombrerete are significantly different from that of all other IAB complex irons, Worsham et al. (2017) suggest that it no longer be classified as a IAB iron. Sombrerete contains 7.3 vol% highly fractionated, rounded silicates, 1–10 mm in size (mostly ~2 mm), located mainly along metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More grain boundaries (Prinz et al., 1982). The silicates show evidence of rapid quenching from a flowing melt, exemplified by the presence of crystal alignment and skeletal crystals. These silicates are highly enriched in alkalis, with compositions ranging from trachybasalt (~48 wt% silicaSilicon dioxide, SiO2.) to alkali-rich basaltic andesite (~55 wt% silica) to andesite (~60 wt% silica) to dacite (~65 wt% silica). The plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More in Sombrerete is unusually Ca-rich compared to that in most other silicated irons, a likely consequence of a fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More process (Ruzicka, 2014). A number of different types of silicate inclusions have been distinguished by Ruzicka et al. (2006). Some inclusions are composed primarily of albitic glass, comprised of equal amounts of plagioclase and quartzComposed of SiO2, quartz is one of the silica group minerals most common in Earth's crust, but never found in meteorites as inclusions visible to the naked eye. Quartz in meteorites has been found in very small quantities in eucrites, other calcium-rich achondrites, and in the highly reduced E chondrites1. Click on Term to Read More with varying amounts of chlorapatite and very fine-grained orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More. Others are composed of glass containing significant amounts of the rare mineral yagiite, a mineral which otherwise has only been reported in the IIE iron Colomera. The occurrence of yagiite infers its crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More from an immiscibly separated K-rich melt (Ruzicka, 2014). Still other types of glass inclusions, which may be Na-, K-, or Na–K-rich, are thought to be derived from immiscibleThe property of liquids that are mutually insoluble (won't mix together) such as oil and water or metallic and silicate melts. Click on Term to Read More melt fractions. These also contain a complex mixture of mineral constituents, including titanean kaersutite, ilmeniteTi-Fe oxide, TiFeO3, found in achondrites, lunar mare basalts, and shergottites. Ilmenite forms as a primary mineral in mafic igneous rocks. It crystallizes relatively early out of a magma before most of the other minerals, and as a result, the heavier crystals of ilmenite precipitate to the bottom of the magma Click on Term to Read More, plagioclase, chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More, merrillite, and tridymiteSilica group mineral in which the tetrahedra occur in sheets. Tetrahedra alternately point up or down to share oxygen with tetrahedra of other sheets, forming six-sided rings perpendicular the sheets. Tridymite has a fairly open structure and accommodates Na+, K+ and Ca2+; charge balance is achieved by Al3+ ↔ Si4+.. Some plagioclase present in inclusions of the latter type exhibits a porous texture (‘spongy’), produced through the crystallization of an immiscible, quartz-enriched melt. Other inclusions of this same type contain P-rich crescent-shaped regions, with orthopyroxene and plagioclase grains showing preferential alignment to these regions suggestive of flow. The metallic host phase is composed of a plessitic intergrowth of kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More and taeniteLess common than kamacite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Taenite, γ-(Fe,Ni), has 27-65 wt% Ni, and forms small crystals that appear as highly reflecting thin ribbons on the etched surface of a meteorite; the name derives from the Greek word for "ribbon." Click on Term to Read More, along with troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More and schreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More. The globular silicate inclusions, considered by some to reflect metal–silicate liquid immiscibility (Prinz et al., 1983; Ruzicka and Hutson, 2003), are now presumed to reflect a filter-press fractionation mechanism (Ruzicka and Hutson, 2005; Ruzicka et al. 2006). Based on their studies, Ruzicka et al. (2006) hypothesize a two-stage formation scenario leading to the observed high fractionation of silicates: Initially, a CR-like chondritic protolith experienced low-degree (~4–8%) partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More as a result of endogenous heating from the decay of short-lived radionuclides. A CR-like protolith is consistent with the measured O-isotopic compositions, as well as the P content of Sombrerete. This partial melting phase produced a phosphoran basaltic andesite. Thereafter, the partially molten metallic host acted as a filter to separate the emergent silicate crystals (primarily chlorapatite and orthopyroxene) from the residual silicate melt as it flowed in-between inclusions. This flow was likely generated by an impact event or a close gravitational interaction, which may have also resulted in the tidal disruption and re-accretion of the planetesimal, thereby separating the solid and molten phases and moving the molten metal–silicate mixture nearer the surface where it was rapidly cooled. A possible period of slower cooling may have followed re-accretion. The compositional variation observed among the silicate inclusions (trachybasalt to dacite) is the result of the variable loss of chlorapatite and orthopyroxene from the Si-poor, P-rich parental liquid. A similar chain of events may have occurred in other silicate-bearing, nonmagmatic irons such as the evolved members of the IIE group, with Colomera showing very close similarities to Sombrerete. The absolute I–Xe age for Sombrerete, calculated relative to Shallowater (4.5623 [±0.0004] b.y.), was determined to be 4.5619 (±0.0010) b.y. (Bogard and Garrison, 2009). This age is considered to reflect the differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More of the silicate and its admixture with the metal phase during parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More disruption. Application of the Hf–W chronometer gave a metal segregation age of 2.1 (±0.9) m.y. after CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More formation (Worsham et al., 2014). This is ~2.4 m.y. earlier than the onset of metal segregation in the IAB main group and members of the sLL subgroup. On the other hand, Worsham and Walker (2016) reported W-isotopic values for Sombrerete that are consistent with those of IAB MG members. Notably, they also determined a W-isotopic composition for sLM subgroup member Persimmon Creek that corresponds to a younger metal –silicate segregation age than that of MG members, which provides support for an impact-melt-pool formation scenario on a common parent body. An Ar–Ar age of 4.541 (±0.012) b.y. was established for Sombrerete, indicating closure occurred only 20 m.y. later than for the I–Xe systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with. However, since it was inferred that no resetting event had occurred since crystallization (Bogard et al., 2000), an Ar–Ar age correction of ~20 m.y. was applied based on improved 40K decay parameters calculated by Vogel and Renne et al. (2008); this correction brings the two chronometers into agreement. These chronometers are therefore most consistent with the scenario of rapid cooling after formation. Both of these ages are older than the corresponding radiometric ages of the IIE irons. The CRE age for Sombrerete was calculated to be 278 m.y. to 819 m.y. (based on 21Ne and 38Ar, respectively), which is also older than that of the IIE irons. The photo shown above is a 49.72 g partial slice of Sombrerete, while the top photo below shows the crusted side. This specimen is from the 433 g section shown in the bottom photo below. The 433 g section was previously part of the J. Schwade Collection, originally obtained from M. Cilz.


Photo courtesy of Dr. J. Piatek
For additional information on collisional dynamics, read the PSRD article by G. Jeffrey Taylor—’Tagish Lake—Hit-and-Run as Planets Formed‘, Nov. 2006.







