Quartz

Composed of SiO2, quartz is one of the silica groupMminerals formed exclusively from silica. There are ten known silica polymorphs, two of which are synthetic. Five of the naturall polymorphs are related by reconstructive transformations and can exist metastably: stishovite, coesite, quartz, tridymite, and cristobalite. Conditions to form coesite and stishovite are attained only during meteoroid impacts where there minerals most common in Earth’s crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More, but never found in meteorites as inclusions visible to the naked eye. Quartz in meteorites has been found in very small quantities in eucritesMost common type of achondrite meteorite and a member of the HED group. Eucrites are basalts composed primarily of pigeonite and anorthite (An60-98). Eucrites have been placed into three subgroups based on mineralogical and chemical differences. • Non-cumulate eucrites represent the upper crust that solidified on a magma ocean after Click on Term to Read More, other calcium-rich achondrites, and in the highly reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More E chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More1.
As reported in the 2018 paper, “First evidence for silica condensation within the solar protoplanetary disk“, the research team found an AOAMillimeter sized, fine-grained inclusions present to a few volume-percent in most carbonaceous chondrites. They can be round but can also be irregularly shaped like an amoeba (thus the name amoeboid). They are forsterite (Mg-rich olivine) and Ca-Al-Ti mineral aggregates. The most characteristic texture of AOAs is an anorthite core (sometimes Click on Term to Read More in the CR2 meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More Yamato 793261 containing typical AOA minerals and ultrarefractory minerals along with grains of quartz (which formed at comparatively lower temperature). These quartz grains measured ∼5 µm in diameter.
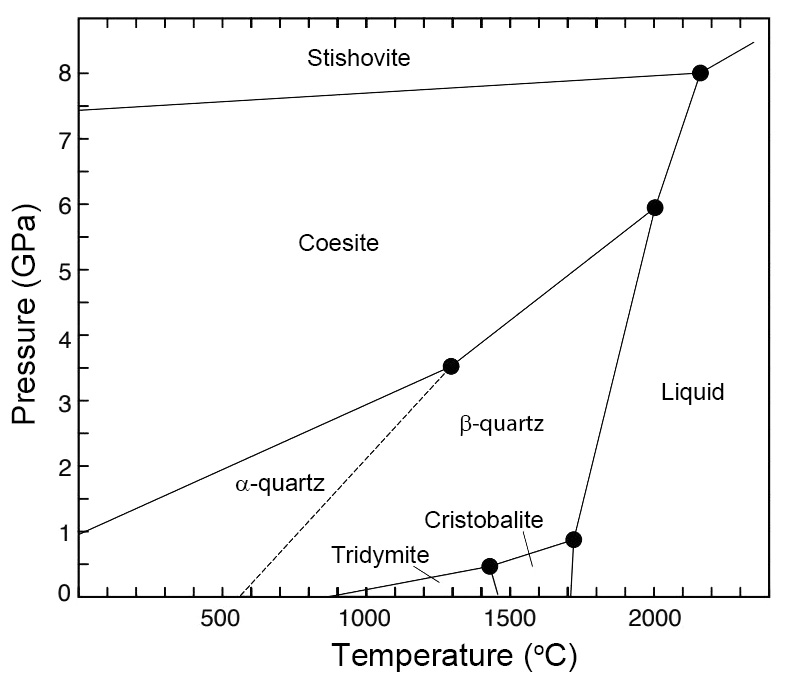
Low-pressure SiO2 polymorphs include quartz (α and β), tridymiteSilica group mineral in which the tetrahedra occur in sheets. Tetrahedra alternately point up or down to share oxygen with tetrahedra of other sheets, forming six-sided rings perpendicular the sheets. Tridymite has a fairly open structure and accommodates Na+, K+ and Ca2+; charge balance is achieved by Al3+ ↔ Si4+. and cristobaliteHigh temperature polymorph of silicon dioxide (SiO2). Has the same chemical composition as coesite, stishovite, seifertite and tridymite but possesses a different crystal structure. This silica group mineral occurs in terrestrial volcanic rocks, martian and lunar meteorites, chondrites and impact glasses like Libyan Desert Glass. Cristobalite has a very open Click on Term to Read More. High-pressure polymorphs include coesiteHigh-pressure polymorph of silicon dioxide (SiO2). Has the same chemical composition as cristobalite, stishovite, seifertite and tridymite but possesses a different crystal structure. Coesite forms at intense pressures of above about 2.5 GPa (25 kbar) and temperature above about 700 °C, and was first found naturally on Earth in impact Click on Term to Read More, stishoviteDense, high-pressure phase of quartz; so far identified only in shock-metamorphosed, quartz-bearing rocks from meteorite impact craters. Stishovite was synthesized in 1961 before it was discovered at Meteor Crater, Arizona. Its structure consists of parallel chains of single SiO6 octahedra. The octahedra are on their sides, sharing opposing edges. Image Click on Term to Read More
Quartz occurs in two forms (α and β) that are related by a displacive transformation. The high-temperature β form is not observed in nature because it converts into the low-temperature α form at 573 °C at 1 kilobar (0.1 GPa) of pressure.
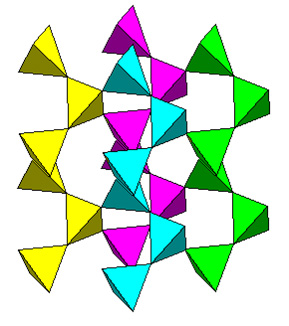
The structure of quartz can be most easily visualized as corkscrewing chains (helices) of silicon tetrahedra aligned along the c axis. The corkscrews take four tetrahedrons to repeat (or three turns in which each tetrahedron essentially rotates 120°). Each helix is connected to two adjacent ones at each tetrahedron.
There is only minor substitutionReplacement of one ion or ionic group for another in the same structural site in a mineral yielding a solid solution. Most substitution in minerals is of cations which are smaller and essentially sit in a lattice of oxygen anions. Anionic substitution does occur in halides. Substitutions are classified based of other elements for Si in quartz. Two trace substitutions postulated for quartz are: (Ti4+)tetrahedral ↔ (Si4+)tetrahedral and (Al3+, Fe3+)tetrahedral + (Fe3+, Na+, Li+, K+)interstitialTerm applied to ions or atoms occupying sites between lattice points. Click on Term to Read More ↔ (Si4+)tetrahedral. However, these substitutions are responsible for many of quartz’s variable colors. For example, amethyst gets its purple-violet color from Fe3+, rose quartz its pink from Ti4+ (and inclusions?), smoky quartz its gray-black color from Al3+, and citrine its yellow from Fe (and irradiation).
Some or all content above used with permission from J. H. Wittke.






