Colony
CO3.0 (3.05)
Found 1975, approx., recognized 1980
35° 21′ N., 98° 41′ W. A single stone weighing 3,912 g was recovered by Deon Yearwood from the tines of his cotton cultivator in Washita County, Oklahoma. The rock was considered to be unusual in appearance and so it was saved. Some years later, as communicated by M. Bostick (2005), Mr. Yearwood asked a professor at Oklahoma University if he would look at the stone to verify his suspicions that it was a meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More. Finding a lack of interest to even look at the rock, he brought it to Southern University at Bedford, Oklahoma, where the suggestion was made to contact Harvey H. Nininger. Upon his initial examination, Nininger immediately recognized the stone as a meteorite, and he and Jim Westcott obtained the meteorite from Mr. Yearwood in return for him receiving two cut slices from the meteorite.
Colony (3.0; 250–260°C)
Kainsaz (3.1; 390°C)
Y-791717 (3.3; 540°C)
Lancé (3.4; 480°C)
Ornans (3.4; 350–560°C)
ALHA77003 (3.5; 500°C)
ALH 83108 (3.5; 570°C)
Isna (3.7; 580–600°C) To discriminate among subtypes below type 3.2, it has been shown that the Cr content of ferroan olivine is an excellent indicator of metamorphism. ChromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More exsolves from olivine in the incipient stages of metamorphism, initially producing heterogeneous Cr2O3 contents, and eventually low-Cr olivine. In a study by Chizmadia and Bendersky (2006), they determined that this sequence progresses from type 3.0, corresponding to high Cr2O3 contents of 0.3–0.4 wt%, to type 3.2, in which Cr2O3 constitutes less than 0.1 wt%. The gap between these subtypes representing type 3.1 was recently filled by the meteorites A-881632 (0.2–0.3 wt% Cr2O3), DOM 03238 (0.27 ±0.18 wt% Cr2O3), NWA 2718 (0.26 ±0.10 wt% Cr2O3), NWA 2760 (0.22 ±0.14 wt% Cr2O3), and NWA 2974 (0.11–0.53 wt% Cr2O3). Previously, petrologic studies revealed that systematic changes occur in AOAs with increasing subtype, which is directly linked to increasing aqueous and thermal metamorphism (Chizmadia et al., 2002). For example, textures and morphologies of AOAs show changes, olivine in AOAs becomes progressively FeO-rich, troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More becomes more prevalent, and trace elements become more equilibrated. Because of their smaller grain size, olivines in AOAs are better indicators of alteration processes (such as the substitutionReplacement of one ion or ionic group for another in the same structural site in a mineral yielding a solid solution. Most substitution in minerals is of cations which are smaller and essentially sit in a lattice of oxygen anions. Anionic substitution does occur in halides. Substitutions are classified based of Fe for Mg) than are the chondrules, which were previously utilized to determine subtype. As a result of their study, Chizmadia et al. (2002) proposed a refinement in the subtypes of the CO3 chondrites; the CO group would span a metamorphic sequence from 3.0, as represented by Colony, to 3.8, as represented by Isna. To make the metamorphic sequence of the CO3 chondrites equivalent to that recognized for the ordinary chondrites, Grossman and Rubin (2006) calibrated the petrologic scale for CO3 chondrites based on the same factors used for ordinary chondrites—the Fa and Cr contents of olivine, and the S content of the matrix (see the Dhofar 015 page for further details). They were able to establish a petrologic sequence consistent with the one utilized for ordinary chondrites, following a progression of metamorphic subtypes in the order 3.00, 3.05, 3.1, 3.15, 3.2, 3.3, etc. They determined that Colony best fits into the 3.05 metamorphic interval. They also demonstrated that the method which utilizes the FeO content of olivine in AOAs (as described above), was only useful in the subtype range of 3.1–3.2. In their studies, Grossman and Rubin determined that the CO3 chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More Dominion Range 03238 fits the requirements for a petrologic type 3.1. Chizmadia and Bravo-Ruiz (2013) employed a similar method to that of Grossman and Rubin (2006) above to classify CO3 chondrites through the entire range by degree of aqueous alteration, utilizing the Fe-Mg composition and distribution in olivines in AOAs. Based on their analyses, they proposed that Colony should be assigned to petrologic type 3.05. They also better resolved Isna as type 3.75, previously designated 3.8, with the MET 00694 pairing group being assigned to the highest CO3 subtype of 3.8. In a different study of CO3 petrologic types conducted by Bonal et al. (2005, 2007), they found that an accurate comparison could be made between the metamorphic grades of the CO and ordinary chondrites using Raman spectroscopy. This methodology is based on various spectral parameters associated with the structural order of insoluble polyaromatic organicPertaining to C-containing compounds. Organic compounds can be formed by both biological and non-biological (abiotic) processes. Click on Term to Read More matter, which was initially accreted in the same proportions in both CO and ordinary chondrites. This structural order is irreversibly transformed by thermal metamorphism (from carbonization to graphitization) to a commensurate degree across chemical classes. A correlation has now been made between this maturation grade of organic matter and the peak metamorphic temperature of the meteorite, and this can then be directly associated with the petrologic grade. To increase accuracy, the Raman shift method was combined with other parameters such as petrographic analyses of phenocrysts in type-I chondrules, including FeO zoning measurements in olivine phenocrysts and Fs compositions in pyroxene phenocrysts (both showing enrichments in higher metamorphic grades), along with petrographic analyses of textures of metal–sulfide associations (e.g., metal–sulfide separation and angularity increases with higher metamorphic grades). In addition, the abundances of presolar grainsMineral grains that formed before our solar system. These tiny crystalline grains are typically found in the fine-grained matrix of chondritic (primitive) meteorites. Most grains probably formed in supernovae or the stellar outflows of red giant (AGB) stars before being incorporated in the molecular cloud from which the solar system Click on Term to Read More (diminished in higher metamorphic grades), noble gasesElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More (P3; diminished in higher grades), and siderophile elements (imprecise indicators) were utilized in their study. Each of these classification parameters were correlated with the degree of thermal metamorphism. From their data, Bonal et al. concluded that the CO group members they studied should span a petrologic sequence as follows: 3.03: ALHA77307
3.1: Colony
3.6: Kainsaz
>3.6: Felix, Lancé, and Ornans (in order of increasing grade)
≥ 3.7: Warrenton and Isna In a further expansion of the Raman spectroscopy method, Quirico et al. (2006) determined that LL3.0 Semarkona has experienced thermal metamorphism beyond the onset stage, and they proposed a new petrologic scale to provide consistency in the range as follows: Semarkona would become petrologic type (PT) 1, with PT 0 being reserved for the stage of true onset of thermal metamorphism. All other meteorites analyzed to date would have a PT greater than 1. Following the scheme of J. Grossman and A. Brearley (2005), the LL chondriteOrdinary chondrites ("low Fe" / "low metal") with only 1 to 3% free metal. Their olivine is more Fe-rich than in the other ordinary chondrites (Fa27-32), implying that the LL types must have formed under more oxidizing conditions than their H or L cousins. Orthopyroxene compositions are also Fe-the rich Click on Term to Read More Semarkona, the L chondriteOrdinary chondrites low in free Ni-Fe metal (4 to 10 vol. %), containing olivine (Fa22-26) and the orthopyroxene hypersthene (Fs19-22). Average chondrule diameters (0.7 mm) are larger than those in H chondrites. The asteroid 433 Eros is suspected as a parent body, based on reflectance spectra, but most L chondrites Click on Term to Read More NWA 7731, and the ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More (probably CO-related; Simon and Grossman, 2015) carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More Acfer 094 (Kimura et al., 2006) were each assigned to the least equilibrated subtype 3.00; however, Semarkona has more recently been determined to represent a petrologic subtype 3.01. For some time ALHA77307 had been considered to be the least metamorphosed CO chondriteMeteorite class named after the Ornans meteorite that fell in France in 1868, are related in chemistry and composition to the CV chondrites and may, with them, represent a distinct clan of carbonaceous chondrites that formed in the same region of the early solar system. However, COs are usually blacker Click on Term to Read More, with an assigned petrologic type of 3.03. A detailed petrographic study of CO3 chondrites was conducted by Davidson et al. (2014, #1384) in order to better define a metamorphic trend for this group. They analyzed the Cr content in olivine of type-II chondrules and identified DOM 08006 as the least metamorphosed CO3 chondrite, assigning it a petrologic type of 3.00. In further support of this low petrologic type assignment, DOM 08006 was determined to have the highest insoluble organic matter (IOM) and C content with the highest H/C, D/H, and 15N/14N ratios among CO3 chondrites, which reflects minimal alteration (Davidson et al., 2014; Alexander et al., 2014, 2017); DOM 08006 also contains the highest abundance of presolar silicates (240 [±25] ppm) as determined by Nittler et al. (2013).

Image courtesy of Schrader and Davidson, GCA, vol. 214, p. 164 (2017)
‘CM and CO chondrites: A common parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More or asteroidal neighbors? Insights from chondrule silicates’ (https://doi.org/10.1016/j.gca.2017.07.031) D.W.G. Sears (2016) conducted an in-depth petrographic study of CO chondrites in an effort to bring a measure of consistency to the wide diversity of classification schemes that now exist for this meteorite group. He studied a significant number of the ‘MIL’ and ‘DOM’ CO chondrites that were found in Antarctica (representing low petrographic types), and updated the petrologic classification of five of the six CO meteorites that were recovered as fresh falls around the world (representing the higher petrographic types). Computer software was used to ascertain which of the many metamorphic properties can best serve as accurate indicators of petrologic type. The results of this ‘Principle Component Analysis’ revealed that 83% of the correlation between metamorphic alteration and petrologic type can be explained by three component types, none of which are decisive when considered alone: 30% is explained by bulk properties (bulk composition, bulk C content, trapped inert gas content, reflectance spectra at 0.8 µm), 28% is explained by metamorphism-induced phase changes (TL sensitivity, matrix composition, graphitization), and 25% is explained by Fe diffusionMovement of particles from higher chemical potential to lower chemical potential (chemical potential can in most cases of diffusion be represented by a change in concentration). Diffusion, the spontaneous spreading of matter (particles), heat, or momentum, is one type of transport phenomena. Because diffusion is thermally activated, coefficients for diffusion Click on Term to Read More processes (olivine composition and heterogeneity, Ni, Co, and Cr content in kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More); the remainder (17%) of the correlation to metamorphic grade can be explained by several less accurate properties such as O- and C-isotopic values and AOA textures. After assessing each of these parameters for the CO chondrites in his study as well as for the known falls (except Moss), a petrologic grade was assigned to each sample (see the following). Based on this CO chondrite study, D.W.G. Sears argues that it is ill-advised to construct a petrologic classification scheme for a common application among different chondrite groups, and he contends that resolution of the metamorphic grade to an accuracy greater than a single decimal place is not warranted for this group given the current techniques. ALHA77307: 3.0
Colony: 3.0
MIL 07099 pairing group: 3.2
DOM 08004 pairing group (excludes DOM 08006): 3.2
Kainsaz: 3.2
Felix, Lancé, and Ornans: 3.4
Warrenton: 3.6
Isna: 3.7 The most unequilibrated CO3 chondrites have isotopic compositions that are similar to anhydrous silicates in the CM group, a group with which it also shares many chemical and petrographic similarities. In fact, the CO and CM groups may represent common precursor material—initially similar to the primitive CO3.00 DOM 08006 and CO3.03 ALHA77307 chondrites—which subsequently experienced different degrees of low-temperature aqueous alteration (Clayton and Mayeda, 1999). Both of these groups probably formed in the same nebular region located beyond 3 AUThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More (Wasson, 1988; Rubin, 2010). Beyond that, new O-isotopic analyses conducted by Greenwood et al. (2014) on a large sampling of CM chondrites led them to suggest that a possible group relationship (same parent body) may exist between the CM and CO chondrites, previously considered to constitute a clan (groups formed at a similar heliocentricCentered around a sun. Our own Solar System is centered around the Sun so that all planets such as Earth orbit around the Sun. Note that 25% of Americans incorrectly believe the Sun revolves around the Earth. Click on Term to Read More distance) based on early research on refractory lithophile abundances, chondrule size and composition, anhydrous mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More compositions, and O-isotopic composition of high-temperature phases (Kallemeyn and Wasson, 1979, 1981). In addition, it was found that the matrix component in meteorites of both groups have nearly identical minor elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More compositions (Greenwood et al., 2014 reference therein). The petrographic and chemical similarities that exist between the CM and CO groups indicate they likely formed from a common reservoir under similar conditions. Further evidence for a common CO–CM parent body was presented by Schrader and Davidson (2016; #1288). They analyzed the Cr content in olivine grain cores of type-II (FeO-rich) chondrules for a number of CM chondrites spanning the full range of petrologic types (e.g., Sutter’s Mill [2.0/2.1]… QUE 97990 [2.6]). Utilizing a coupled diagram comparing the mean Cr2O3 content to the standard deviation (σ) of Cr2O3 content, they demonstrated that both the CO and CM thermal metamorphism curves overlap. Their study also shows that thermal metamorphism and aqueous alteration are not coupled. Another coupled diagram presented by Schrader and Davidson (2016) comparing the Fe and Mn contents of the type-II chondrules among the CM samples also demonstrates significant overlap which is consistent with a common CO–CM parent body. Nevertheless, Schrader and Davidson (2017) recognize multiple lines of evidence which indicate these two groups derive from separate parent bodies including the following:
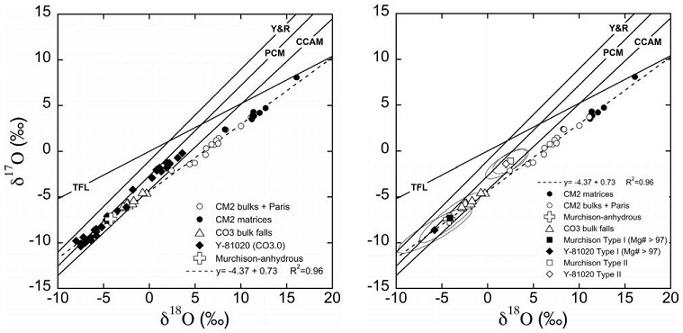
- difference in Δ17O and ε54Cr values (Sanborn et al., 2014 [see diagram])
- difference in matrix abundance (CM: 70 wt% vs. CO: 34 wt%; Weisberg et al., 2006)
- difference in average chondrule diameter (CM: 0.3 mm vs. CO: 0.15 mm; Weisberg et al., 2006)
- difference in group average 21Ne-based CRE ages (CM: 2.8 [±3.1] m.y. vs. CO: 22 [±18] m.y.; Mazor et al., 1970 [see diagram])
- difference in FeO-poor relict grains (CM: ~12% vs. CO: ~48%; Schrader and Davidson, 2017)
- no known CM/CO meteorite breccias (Schrader and Davidson, 2017)
In their comprehensive oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More isotope study of carbonaceous chondrite groups, Clayton and Mayeda (1999) showed that many ungrouped members plot along the same mixing line and fill the hiatus between the CO and CM fields (see diagram below). They suggest that both CO and CM groups consist of a common anhydrous silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the precursor, while the CM group represents the interaction of this anhydrous precursor with an aqueous reservoir. The ungrouped members are transitional, with variable water:rock ratios as indicated by the tick marks along the mixing line. 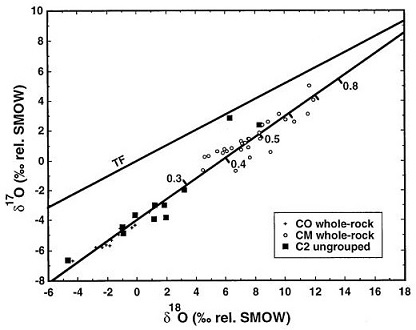
Image courtesy of Clayton and Mayeda, GCA, vol. 63, p. 2094 (1999)
‘Oxygen isotope studies of carbonaceous chondrites’
See also this oxygen three-isotope diagram presented by Jacquet et al., MAPS, vol. 51, #5, p. 862 (2016)
‘Northwest Africa 5958: A weakly altered CM-related ungrouped chondrite, not a CI3’ (http://dx.doi.org/10.1111/maps.12628) Although there is a hiatus between the CM and CO groups on an oxygen three-isotope diagram, the additional data plots calculated by Greenwood et al. (2014) clearly show that the CM O-isotopic trend line intersects the CO field, and they have posited a new theory based on the premise that both groups formed on a common parent body. They suggest that the CO group might represent an anhydrous inner zone, in which the initial hydrous component was rapidly liberated through endogenous (radiogenic) heating and vented to the surface and into space. Conversely, the outer zone represented by the CM group experienced a high degree of aqueous alteration over an extended duration. A compatable scenario was presented by Fu and Elkins-Tanton (2013) in which early accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More (within ~2 m.y. of CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More formation) of a planetesimal of significant size (>120 km in diameter), composed of low-density material akin to the CM chondrites, could experience internal differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More without eruption of magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More to the surface, thereby retaining a primitive hydrated crustal region. 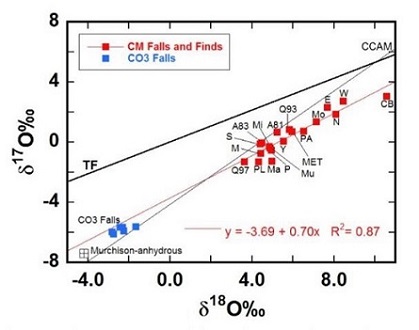
Diagram courtesy of Greenwood et al., 45th LPSC #2610 (2014)
A81:ALHA81002; A83:ALH 83100; CB:Cold Bokkeveld; E:Essebi; Ma:Maribo; MET:MET 01070; MI:Mighei; Mo:Moapa; M:Murchison; Mu:Murray; N:Nogoya; P:Paris (mean); PA:Paris-altered; PL:Paris-less altered; S:SCO06043; Q93:QUE93005; Q97:QUE97990; Y:Y791198; W:WIS91600; CO3 falls:Moss A comparitive analysis of CM and CO chondrites led Chaumard et al. (2018) to the conclusion that both of these groups formed in a common isotopic reservoir and accreted identical anhydrous precursor material comprised of the same two type I chondrule populations: 1) Δ17O ~ –2.5‰, Mg# <96, and 2) Δ17O ~ –5‰, Mg# >98.5. Both CM and CO chondrites also accreted identical type II chondrule populations. However, they recognized the many other characteristics that indicate a formation for these two groups on separate parent bodies, including differences in chondrule size (0.15 and 0.30 mm for CO and CM, respectively), matrix abundance (30–35 and 70 vol% for CO and CM, respectively), abundance of type II chondrules that contain relict olivine grains (~48% and 12–25% for CO and CM, respectively), average CRE age (22 [±18] and 2.8 [±3.1] m.y. for CO and CM, respectively), accretion age (~2.1–2.7 and ~3.5–5.0 m.y. after CAIs for CO and CM, respectively), and in abundance of hydrous phases (ice:rock ratio of ~0.1–0.2 and ~0.3–0.6 for CO and CM, respectively). They propose a scenario in which the snow line moved inward during the time interval between the accretion at nearly the same location (~2–3 AU) of these two distinct planetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More.
Diagram credit: Chaumard et al., GCA, vol. 228, p. 220–242 (1 May 2018)
‘Oxygen isotope systematics of chondrules in the Murchison CM2 chondrite and implications for the CO-CM relationship’
(https://doi.org/10.1016/j.gca.2018.02.040) Kimura et al. (2008) argue for the additional inclusionFragment of foreign (xeno-) material enclosed within the primary matrix of a rock or meteorite. Click on Term to Read More of CR, CH, CB, and CM carbonaceous chondrites as petrologic type 3.00 examples, notwithstanding the general designation of some meteorites in these groups as type 2 due to aqueous alteration features. In light of this petrologic typing paradox, they propose that a separate scale be adopted to describe aqueous alteration distinct from that which describes thermal metamorphism. Separate classification schemes for aqueous alteration have since been proposed (see the Murchison page for details.
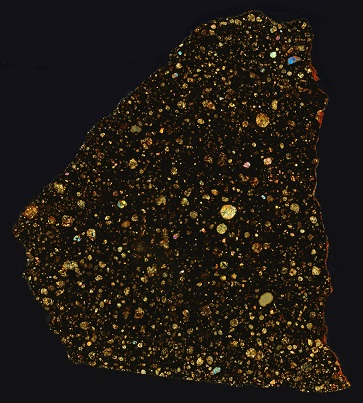
click on image for a magnified view
Photo courtesy of Peter Marmet