NWA 7325
AchondriteAn achondrite is a type of stony meteorite whose precursor was of chondritic origin and experienced metamorphic and igneous processes. They have a planetary or differentiated asteroidal origin where the chondritic parent body reached a sufficient size that through heating due to radioactive decay of 26Al (aluminum isotope) and gravitational Click on Term to Read More, ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More
Found in 2012
no coordinates recorded Thirty-five separate pieces of a meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More having a combined weight of 345 g, some exhibiting a unique and attractive chartreuse-colored fusion crustMelted exterior of a meteorite that forms when it passes through Earth’s atmosphere. Friction with the air will raise a meteorite’s surface temperature upwards of 4800 K (8180 °F) and will melt (ablate) the surface minerals and flow backwards over the surface as shown in the Lafayette meteorite photograph below. Click on Term to Read More, were found near a well known by local hunters as Bir Abbass, located near Asrifa, Morocco (per H. C. Aoudjehane). These stones were subsequently purchased by S. Ralew in April 2012 from a dealer in Erfoud, Morocco. A type sample was submitted by Mr. Ralew to the University of Washington in Seattle (A. Irving and S. Kuehner) for analysis and classification and NWA 7325 was determined to be a new ungrouped achondrite. Numerous additional paired fragments from this fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More have since been recovered, including 210 g NWA 8014 acquired by N. Jain, 3.23 g NWA 8268 acquired by R. Lenssen, 82.37 g NWA 8353 acquired by S. Perekslis, numerous stones totalling 433.9 g constituting NWA 8409 (main mass photo on poster by Hoffmann et al., 2018; high-res interior view courtesy of Peter Marmet), 43.81 g NWA 8486 acquired by R. Garcia, and many visually obvious pairings not yet analyzed and given a NWA-series designation (e.g., larger masses weighing 211 g, 86 g, and 43 g (NWA 8486?); a 27.83 g stone exceptionally photographed in high-res by M. Ouzlou showing the true color and texture of the unique fusionProcess in which two lighter atomic nuclei combine to form a heavier atomic nucleus. Very high temperatures are normally required in order for atomic nuclei to collide with sufficient energy to overcome the Coulomb barrier (their mutual electrostatic repulsions). Fusion that occurs under high-temperature conditions is called thermonuclear fusion. Fusion Click on Term to Read More crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More; plus obvious smaller paired stones acquired by Morrocan ebay seller ‘ayoubhaddououmoussa’ weighing 10 g, 7.5 g, 2 g, and 2 g).
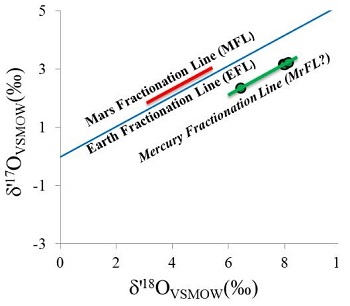
Diagram credit: Jabeen et al., 45th LPSC, #2215 (2014) The mineralogical and geochemical properties of NWA 7325 are consistent with accumulation from a Ca-rich and Fe-poor maficOne of the two broad categories of silicate minerals, the other being felsic, based on its magnesium (Mg) and/or iron (Fe) content. Mafic indicates silicate minerals that are predominantly comprised of Mg and/or Fe.The term is derived from those major constituents: Magnesium + Ferrum (Latin for iron) + ic (having Click on Term to Read More magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More under highly reducingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions. Sutton et al. (2014) utilized X-ray absorptionTransfer of energy to a medium as a particle or electromagnetic radiation passes through it. Absorption of electromagnetic radiation is the combined result of Compton scattering, σ, and photoelectric absorption, τ. It may be quantified: where, t = thickness, ρ = density, and μ = mass absorption coefficient, which combines Compton and photoelectric effects (μ = σ + τ). Click on Term to Read More spectroscopy for elemental analyses of olivine and pyroxene in NWA 7325 to discover the valency (oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More state) of the Ti, V, and Cr. Based on the oxidation state of these multivalent elements relative to the iron-wüstite (Fe-FeO) redoxOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More buffer assemblage, the oxygen fugacityUsed to express the idealized partial pressure of a gas, in this case oxygen, in a nonideal mixture. Oxygen fugacity (ƒO2) is a measure of the partial pressure of gaseous oxygen that is available to react in a particular environment (e.g. protoplanetary disk, Earth's magma, an asteroid's regolith, etc.) and Click on Term to Read More of the primary melt during crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More of NWA 7325 was estimated to be ~IW-4.5; these highly reducing conditions are consistent with the redox state of the inner Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids.. While that may be true, results of high precision trace element analyses conducted by Weber et al. (2015) reveal some inconsistencies with a formation of NWA 7325 under reducing conditions; e.g., higher depletions of W in the silicates would be expected, resulting in higher Ta/W and Hf/W values compared to those observed. They suggest that the introduction of a metasomatic fluid (partial melt phase) with a high concentration of highly incompatible elements such as W and Nd (both due to their high field strength) could explain these low values as well as the unusually low Zr/Nb and Sm/Nd values. In an effort to better characterize the redox conditions under which NWA 7325 was formed, Sutton et al. (2017) determined the valences for a number of multivalent elements (Ti, Cr, and V) which can be informative of the oxygen fugacity at the time of silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the igneous crystallization. By employing these three elements in combination, coverage of the oxygen fugacity can be achieved over a broad range (~ IW–11 to IW+4) consistent with that considered to have prevailed on the parent bodies of achondrites. Results of their study demonstrated that all three oxybarometers in olivine grains, as well as the Ti and V oxybarometers in pyroxene grains, are indicative of highly reducing conditions during formation of both NWA 7325 and the ureilite Y-791538 equivalent to a combined weighted mean of IW–3.1 (±0.2) and IW–2.8 (±0.2), respectively; notably, this oxygen fugacity value also falls within the broad range (~IW–6 to –3) deduced by McCubbin et al. (2012) for Mercury based on MESSENGER data. Sutton et al. (2017) posited that the more oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More Cr (IW+1) present in the pyroxene and plagioclase grains in NWA 7325 is attributable to a secondary shock-heating event. Hasegawa et al. (2014) suggested that the parental source melt composition for NWA 7325 was akin to enstatite chondriteType of meteorite high in the mineral enstatite and also referred to as E-chondrites. Although they contain substantial amounts of Fe, it is in the form of Ni-Fe metal or sulfide rather than as oxides in silicates. Their highly reduced nature indicates that they formed in an area of the Click on Term to Read More with an added CAI-like component; however, Goresy et al. (2013) recognized a unique REEOften abbreviated as “REE”, these 16 elements include (preceded by their atomic numbers): 21 scandium (Sc), 39 Yttrium (Y) and the 14 elements that comprise the lanthanides excluding 61 Promethium, an extremely rare and radioactive element. These elements show closely related geochemical behaviors associated with their filled 4f atomic orbital. Click on Term to Read More pattern and positive Eu anomaly associated with possible residual oldhamiteMn-Ca sulfide, (Mn,Ca)S, is a pale to dark brown accessory mineral found in minor amounts in highly reduced meteorites such as many enstatite chondrites, and some aubrites and enstatite achondrites. Oldhamite in enstatite chondrites likely formed by solar nebular gas condensation. CaS Oldhamite was also found in the most fresh Click on Term to Read More, which would rule out derivation from any enstatiteA mineral that is composed of Mg-rich pyroxene, MgSiO3. It is the magnesium endmember of the pyroxene silicate mineral series - enstatite (MgSiO3) to ferrosilite (FeSiO3). Click on Term to Read More chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More or aubriteAubrites are named for the Aubres meteorite that fell in 1836 near Nyons, France. They are an evolved achondrite that is Ca-poor and composed mainly of enstatite (En100) and diopside (En50Wo50) with minor amounts of olivine (Fa0) and traces of plagioclase (An2-8). They contain large white crystals of enstatite as Click on Term to Read More. MESSENGER X-ray (XRS) and gamma-ray (GRS) fluorescenceEmission of electromagnetic radiation, especially of light, that results from bombardment of a substance with other forms of electromagnetic radiation. This spontaneously emitted radiation ceases immediately after excitation ceases. Click on Term to Read More data analyzed by Nittler et al. (2013) indicate that significant compositional variation in Fe and other elements exists across the surface of Mercury (irrespective of a still incomplete global coverage, particularly of the northern hemisphere), but on average the near-surface bulk composition of the planet is relatively poor in Fe (<~4 wt% [~0.2–~1.5 wt%]; Izenberg et al., 2013), Ti (<1 wt%), and Al; these average values correspond most closely to plains units. Furthermore, near-infrared reflectance spectra obtained from the Visible and Infrared Spectrograph (VIRS) instrument aboard the spacecraft, attaining a planetary coverage of >90% plus specific fresh craters (Klima et al., 2013), indicate that the FeO content of surface silicates (primarily Mg-rich orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More and plagioclase; Stockstill-Cahill et al., 2012) can be constrained below ~2 wt% (ave. ~1 wt%; Weider et al., 2013); this value is in broad agreement with the 1.57 wt% FeO constituting this meteorite (Irving et al., 2013). Other XRS and GRS data utilized by Vaughan et al. (2013) indicate that a relatively high abundance of S (and certain other volatile elementsChemical elements that condense (or volatilize) at relatively low temperatures. The opposite of volatile is refractory. Volatile elements can be divided into moderately volatile (Tc = 1230–640 K) and highly volatile (Tc < 640 K). The moderately volatile lithophile elements are: Mn, P, Na, B ,Rb, K, F, Zn. The moderately Click on Term to Read More) exists across Mercury’s surface, contrary to favored theoretical predictions, with measurements showing up to ~4 wt% (ave. ~2 wt%) (Evans et al., 2013). Given the hypothesized formation environment that existed on Mercury, a high probability was found for about half of the S abundance to occur as FeS—Fe being the limiting factor—with the remainder occurring as CaS (oldhamite) without MgS. Another Ni-bearing iron component is believed to exist on the surface as free metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More derived from impact debris. The very low abundance of FeO on the surface is thought to reflect the sequestration of Fe under very reducing conditions and high temperatures into the unusually large core (>50 vol%). In a similar manner, it is argued by Hillgren and Fei (2013) that Si would likely experience a mutually high sequestration into the core under these conditions, leaving a high S abundance in the mantle. An experiment was conducted by Vaughan et al. (2013) in an effort to synthesize the products of a mercurian magma. The batch contained the elements in the proportions detected in the bulk composition of surface material on Mercury by XRS. The experiment was run under the hypothesized formation conditions that existed including temperature, pressure, redox, and cooling rate. The resulting mineral assemblage included forsterite, diopside, and sulfides, the latter including FeS and CaS but no MgS. Relatively high abundances of Ca are present in NWA 7325, with a significant occurrence of residual oldhamite being reported by Goresy et al., 2014). However, this Ca-sulfate component was interpreted by Barrat et al. (2014, 2015) as more likely representing a product of terrestrial weathering. XRS and GRS values for Ca from partial coverage of the planet’s surface are relatively low (although higher values are present in certain regions; Nittler et al., 2013), suggesting a general low abundance of plagioclase minerals (Weider et al., 2011, 2013). In a like manner, Evans et al. (2013) analyzed the data as it pertains to volatile abundances on the surface, finding variable Na abundances across the planet—higher in the smooth plains (3.9–5.2 wt%) compared to the heavily cratered terrain (0.7–2.9 wt%). Alternatively, the high and low Na abundances might instead correspond to the cooler polar regions and warmer equatorial regions, respectively. Both telescopic observations and petrologic modeling of Mercury’s crust suggest that there should be a significant component of Na-rich plagioclase in some regions, although none has been reported thus far in NWA 7325. It was argued by some that any significant discrepancies that may exist between the expected abundances of Ca and Ca-bearing minerals on Mercury’s surface, compared to the actual abundances measured in NWA 7325, may be mitigated by a scenario in which the meteorite formed at significant depth involving fractionation processes in the parent melt, and/or it formed in a highly reducing environment. Other considerations may be invoked to account for the lack of sodic plagioclase in this meteorite. Further investigation is also needed to account for the apparent heterogeneity in elemental abundance ratios which exists across the planet as demonstrated by many research teams (e.g., Izenberg et al., 2013). Modeling of the parent body of NWA 7325 was conducted by Archer et al. (2015) in consideration of the observed low abundances and unique fractionation profile of highly siderophile elements (HSE), as well as the Re–Os isotopic data. The HSE characteristics of the meteorite samples are inconsistent with a simple one-stage model involving metal-silicate segregation during core formation, but a multi-stage formation scenario can produce a very similar HSE signature to that of the samples studied. It was proposed that initial melting and differentiation of a chondritic body resulted in a 70:30 compositional ratio comprising silicate mantle and FeNi-metal core, respectively. Thereafter, late accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More and incorporation of a small exogenous chondritic component was succeeded by an equilibration event. Then ensued another episode of metal-silicate segregation, followed by lithification of the meteorite source rock. A final addtion of a small volume of CI-like chondritic material ultimately resulted in a rock with HSE characteristics very similar to those of the NWA 7325 meteorite fragments used in this study. Based on petrographic, chemical, and isotopic analyses of NWA 7325, Barrat et al. (2015) proposed an alternate formation scenario. In consideration of multiple features inherent in the meteorite, including heterogeneous HSE distribution, very low abundances of incompatible elements, very large Eu anomaly, and high δ26Mg* value, and with regards to the Al–Mg systematics for the meteorite, they concluded that the meteorite represents in situ crystallization of an impact melt rather than a cumulate derived from a melt. This impact-melting and crystallization event occurred ~4.5628 b.y. ago from a gabbroic host protolith calculated to have formed ~4.566 b.y. ago. In contrast to the scenario presented by Archer et al. (2015) described above, and more consistent with the unbrecciated nature of NWA 7325, Barrat et al. (2015) argue that the addition of the small exogenous chondritic component (≈0.2 wt%) occurred prior to crystallization of the meteorite source protolith. Through chronometric studies of NWA 7325 based on the U–Pb isotopic systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with, Amelin et al. (2013) ascertained an ancient age of 4.5625 (±0.0044) b.y. The Al–Mg age for NWA 7325 relative to the D’Orbigny angriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More was calculated by Dunlap et al. (2014) to be 4.5628 (±0.0003) b.y., which matches the Pb–Pb chronometer. The importance of an accurate and precise determination of the crystallization age for this unique achondrite led Koefoed et al. (2016) to conduct careful analyses of the U–Pb and Al–Mg systems. Employing the precise 238U/235U value of 137.794 (Goldman et al., 2015), their most reliable Pb–Pb isochron age is 4.5634 (±0.0026) b.y., while that for the Al–Mg chronometric system anchored to D’Orbigny was found to be accordant, providing a very precise determination of the crystallization age at 4.56309 (±0.00026) b.y., or 4.2 m.y. after CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More. A larger sample of the paired meteorite NWA 8486 was analyzed by Cartwright et al. (2016) in order to better resolve the U–Pb age. They derived a best age of 4.5639 (±0.0017) b.y. In addition, Koefoed et al. (2016) utilized Al–Mg systematics to estimate the timing of differentiation of the meteorite parental source melt, establishing that this stage occurred within 1.72–1.80 m.y. after CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More formation. The unusually high abundance of radiogenic Al inferred to be present at the time of crystallization has implications for its distribution in the early solar system, and helps to establish that NWA 7325 originated on a unique parent body very early in solar system history. Additional age dating of NWA 7325 based on Ar chronometry was conducted by Hopp et al. (2018). They derived an Ar–Ar age, corrected for the accumulated terrestrial atmospheric argonNoble gas represented by the atomic symbol Ar, that has Z = 18, and an atomic weight of 39.948. It is colorless, odorless, and very inert gas, comprising ~1 % of the Earth's atmosphere. Click on Term to Read More component, of 3.200 (±0.260) b.y.; this likely represents an upper age limit for a late-stage impact-induced degassing event. The 3He and 21Ne cosmogenic noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More data of Hasegawa et al. (2014) provide a CRE age for NWA 7325 of ~20 m.y., which is consistent with the Xe- and Ar-based CRE age determined by Weber et al. (2015) of 18.8–18.9 m.y. A precise calculation process was employed by Hopp et al. (2018) to better resolve the CRE age based on cosmogenic 38Ar, and an age of 17.45 (±0.12) m.y. was derived. This age is seemingly in accord with the age determined by Weber et al. (2015), but actually the two ages are at variance when the same calculation procedures are used—the Weber et al. (2015) age would then be 13.5–14 m.y. After a thorough assessment, the CRE age discrepancies could only be attributed to compositional heterogeneity with respect to noble gasesElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More. The finding of such a very ancient crystallization age for this meteorite runs counter to expectations for an origin on a planet the size of Mercury, as this is an age intuitively expected for a relatively small, rapidly cooled asteroid (Dunlap et al., 2014). Furthermore, this crystallization age is indistinguishable from that calculated for other ungrouped achondrites such as NWA 011 and NWA 6704 (Koefoed et al., 2016). That said, there have been very ancient crustal samples (or components therein) delivered to Earth from the larger planet Mars; until better knowledge of the formation history of Mercury is obtained, a genetic relationship with NWA 7325 should not be ruled out. A report on the Xe-isotopic systematics of NWA 7325 describes an elevated 129Xe component considered likely to be from the radioactive decayProcess in which an isotope's nucleus changes ('decays') to produce another isotope. The original atom is called the 'parent' and the resulting atom, the 'daughter'. There are three modes of radioactive decay: • Emission of a particle (He nucleus) that decreases the atomic number (Z) by 2 and the atomic Click on Term to Read More of 129I (Crowther et al., 2014; Crowther and Gilmour, 2015). This elevated 129Xe could be the result of the decay of primary 129I (half-lifePeriod of time required for 50% (½) of the atoms of a radioactive nuclide in a sample to decay. After two half-lives, 25% ( ½ x ½ = 1/4) of the original radioactive nuclide will remain. After three half-lives, 12.5% ( ½ x ½ x ½ = 1/8) of the original radioactive nuclide will remain. Click on Term to Read More = 16 m.y.) that was incorporated early in Solar System history, or possibly it was acquired much later from a reservoir containing an evolved Xe component; the latter case would require heating and then closure of this chronometer after several 10s of million years had passed, possibly associated with the secondary heating and melting event inferred from petrographic analyses (e.g., Weber et al., 2015). Additional constraints on the origin of this meteorite have been established through studies of the Cr-isotopic systematics (Sanborn et al., 2013, 2014; Kita et al., 2014). The resulting ε54Cr value of –0.55 (±0.08) resolves NWA 7325 from both the ureilites (ε54Cr ~ –0.9; Yamakawa et al., 2010) and the acapulcoite–lodranite clan (ε54Cr = –0.75; Göpel and Birck, 2010)—groups for which discrimination through O-isotopic values alone had not been attained. In a similar view, mineralogical and trace element studies conducted by Goresy et al. (2014) provide further evidence against a genetic relationship between NWA 7325 and either the ureilites or the enstatite chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More and aubritesAubrites are named for the Aubres meteorite that fell in 1836 near Nyons, France. They are an evolved achondrite that is Ca-poor and composed mainly of enstatite (En100) and diopside (En50Wo50) with minor amounts of olivine (Fa0) and traces of plagioclase (An2-8). They contain large white crystals of enstatite as Click on Term to Read More. Sanborn and Yin (2014) compared various meteorites utilizing a ε54Cr vs heliocentricCentered around a sun. Our own Solar System is centered around the Sun so that all planets such as Earth orbit around the Sun. Note that 25% of Americans incorrectly believe the Sun revolves around the Earth. Click on Term to Read More distance coupled diagram (see below). The ε54Cr value of –0.55 (±0.08) calculated for NWA 7325 does not plot in the present location expected for Mercury, unless the planet is considered to have formed at a greater heliocentric location before migrating inward.
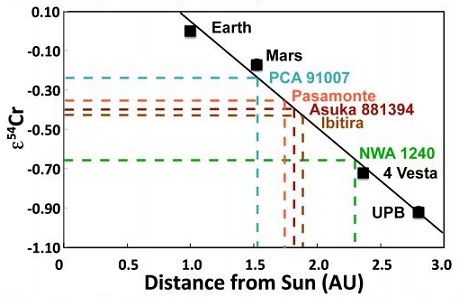
Diagram credit: Sanborn and Yin, 45th LPSC, #2018 (2014) Interestingly, inward migration follows the scenario proposed by Asphaug and Reufer (2014) in light of their hydrocode simulation of a hypothetical Mercury-forming hit and run collision (see below). They theorized that a differentiated chondritic impactor (proto-Mercury) having 25% the mass of Earth could have collided with proto-Venus at a given velocity and angle so that the mantle of the impactor was stripped and dispersed, leaving a largely metallic secondary body which matches the mass (5.5% the mass of Earth) and composition (~70 mass% metallic iron) of Mercury. Further modeled data pertaining to this hypothetical collision can be found in the The Astrophysical Journal article ‘Formation of the Terrestrial PlanetsRocky planets: Mercury, Venus, Earth, and Mars. These planets have physical characteristics, chemical composition and internal structure similar to the Earth. The terrestrial planets have 0.4% of the total mass of all the planets in the Solar System. Some large satellites of planets are also similar to the characteristics of from a Narrow Annulus’, Section 3.1. Origin of Mercury, by B. Hanson (2009). Simulation of a hypothetical Mercury-forming hit and run collision from Erik Asphaug. As expressed by Sanborn et al. (2014), a coupled Δ17O vs. ε54Cr diagram is one of the best diagnostic tools for determining genetic relationships between meteorites. Moreover, Sanborn et al. (2015) demonstrated that ε54Cr values are not affected by aqueous alteration. The diagram below, shown courtesy of Goodrich et al. (2017), compares the Δ17O and ε54Cr isotopic composition of NWA 7325 with other achondrite and carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More groups.
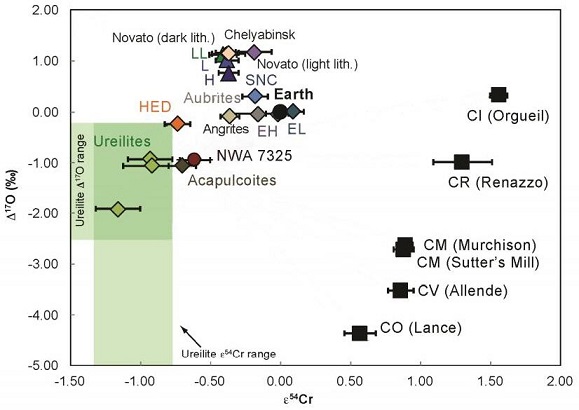
Diagram adapted from Goodrich et al., GCA, vol. 203, p. 395 (2017)
‘Petrogenesis and provenance of ungrouped achondrite Northwest Africa 7325 from petrology, trace elements, oxygen, chromium and titanium isotopes, and mid-IR spectroscopy’
(http://dx.doi.org/10.1016/j.gca.2016.12.021) In their study of the fossil meteoriteThe textural, mineralogical or compositional remnant within a sedimentary rock of a meteorite that fell millions of years ago and found in Ordovician limestone from Sweden. Read Tiny Traces of a Big Asteroid Breakup for a complete writeup on this subject. In picture to the left a nautiloid shell is Click on Term to Read More Österplana 065, Schmitz et al. (2016) included oxygen and chromium isotopic data for NWA 7325 in a coupled Δ17O vs. ε54Cr diagram (shown below). This higher resolution diagram clearly demonstrates that this meteorite plots in a distinct location in isotope space, indicating that it derives from a unique parent body. Chromium vs. Oxygen Isotope Plot
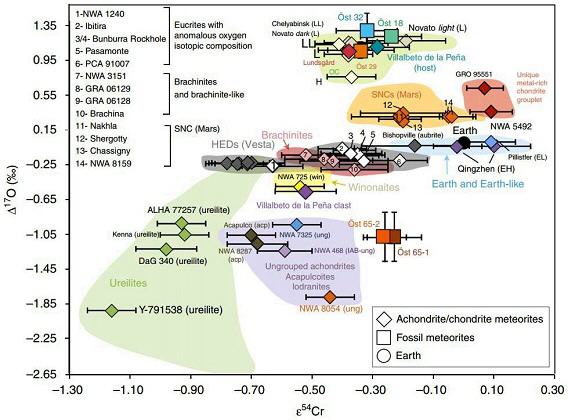
click on image for a magnified view Diagram credit: Schmitz, B. et al., Nature Communications, vol. 7, p. 4 (2016, open access link)
‘A new type of solar-system material recovered from Ordovician marine limestoneA common form of calcium carbonate (CaCO3). Other common forms of CaCO3 include chalk and marble. Click on Term to Read More ‘
(https://doi.org/10.1038/ncomms11851) Microprobe results for trace element abundances in NWA 7325 show depletions in highly incompatible elements (Kita et al., 2014). It was noted that close similarities exist between element depletions in both NWA 7325 and an An-rich plagioclase clastA mineral or rock fragment embedded in another rock. Click on Term to Read More found in the DaG 319 polymict ureilite. It was argued that a low-degree partial melt phase, which was responsible for the sequestration of highly incompatible elements from both NWA 7325 and the An-rich plagioclase clast, preceded the formation of both lithologies on their respective parent bodies. Furthermore, it was suggested that both of these planetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More experienced low-temperature aqueous processing prior to differentiation, which was made possible by abundant accreted water ice that is most prevalent in the outer solar system; therefore, it was asserted that NWA 7325 likely originated on an asteroid similar to the ureilite parent body with which it shares some similarities in petrogenetic history. To date, six feldspathic clasts have been identified in polymict ureilites (see further details on the DaG 319 page) which have mineral and O-isotopic compositions as well as fine textures remarkably similar to NWA 7325 (Goodrich et al., 2017). They consider it plausible that these clasts represent xenolithic fragments derived from the NWA 7325 parent body. Further comparisons utilizing a possible second sampling of the NWA 7325 parent body, the 453 g NWA 11119 (photo courtesy of Carl Agee; see also photos below), should be elucidating. Results of petrologic and isotopic analyses of NWA 11119 conducted by Srinivasan et al. (80th MetSoc, 2017, #6129; Nature Communications, 2018, #3036) reveal that this meteorite has essentially identical O-isotopic values to NWA 7325, both are depleted in alkalis, and both contain a high abundance of calcic plagioclase and Cr-bearing clinopyroxene (bright green diopside). Utilizing a coupled Δ17O vs. ε54Cr diagram, Huyskens et al. (2018) demonstrated that the two meteorites overlap within uncertainties (see diagram below below). Unlike NWA 7325 however, NWA 11119 has an andesitic–dacitic bulk composition, has a unique Fe/Mn ratio (indicative of an origin on a previously unsampled parent body), is significantly more oxidized, lacks olivine and metal, and contains a uniquely high abundance (~22%) of silicaSilicon dioxide, SiO2. polymorph in the form of tridymiteSilica group mineral in which the tetrahedra occur in sheets. Tetrahedra alternately point up or down to share oxygen with tetrahedra of other sheets, forming six-sided rings perpendicular the sheets. Tridymite has a fairly open structure and accommodates Na+, K+ and Ca2+; charge balance is achieved by Al3+ ↔ Si4+. (Srinivasan et al., 2018). In addition to tridymite, cristobaliteHigh temperature polymorph of silicon dioxide (SiO2). Has the same chemical composition as coesite, stishovite, seifertite and tridymite but possesses a different crystal structure. This silica group mineral occurs in terrestrial volcanic rocks, martian and lunar meteorites, chondrites and impact glasses like Libyan Desert Glass. Cristobalite has a very open Click on Term to Read More and rare quartzComposed of SiO2, quartz is one of the silica group minerals most common in Earth's crust, but never found in meteorites as inclusions visible to the naked eye. Quartz in meteorites has been found in very small quantities in eucrites, other calcium-rich achondrites, and in the highly reduced E chondrites1. Click on Term to Read More was reported in Hoffmann et al., 2018, #2468). Both its fine-grained vesicularVesicles appear in nature when they are produced within lava (extrusive aphanitic igneous rock) whose dissolved gases come out of solution (are released) due to the drop in pressure during an eruption. The resulting lava solidifies around the gas bubbles capturing their shape inside and outside the rock. Vesicles do Click on Term to Read More texture and its silica-rich composition are consistent with an evolved lavaHot molten or semifluid rock derived from a volcano or surface fissure from a differentiated and magmatically active parent body. Click on Term to Read More associated with a relatively early (within ~3 m.y. of solar system history) volcanicIgneous rock that forms from cooling magma on the surface of a planet or asteroid. eruption. Based on Al–Mg chronometry, Srinivasan et al. (2018) calculated an age of 4.5648 (±0.0003) b.y. for NWA 11119 relative to the D’Orbigny angriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More. This age is nearly 2 m.y. (~1.7 m.y. based on replicate data) older than that calculated for NWA 7325 and is among the oldest determined for a meteorite thus far. Notably, Wimpenny et al. (2019) calculated an Al–Mg age for the anomalous A-881394 of 4.56483 (±0.00021) b.y. when anchored to the D’Orbigny angrite, while an age up to 1.26 m.y. younger was obtained when anchored to certain CAIs. They also calculated a similar Mn–Cr age for A-881394 of 4.56441 (±0.00032) b.y. anchored to D’Orbigny. In addition, employing the measured 238U/235U value of 137.768 (±0.038), they determined the most precise and accurate Pb–Pb isochron age for A-881394 (possibly related to Bunburra Rockhole) to be 4.56495 (±0.00053) b.y.; this is the oldest meteorite dated utilizing U–Pb chronometry.
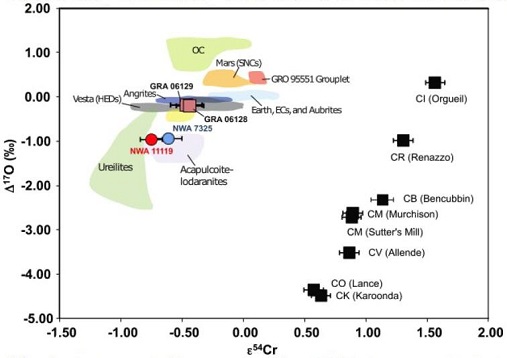
Diagram credit: Huyskens et al., 49th LPSC, #2311 (2018)

NWA 11119 photo courtesy of Aziz Habibi

click on photo for a magnified view
NWA 11119 photos courtesy of Karim Abdelvetah

NWA 11119 showing green fusion crust and interior
Photo credit: © 2017 B. Barrett/Maine Mineral & Gem Museum
See a YouTube video examination of a hand sample of NWA 11119 by Carl Agee at this link In their study of NWA 11119, Srinivasan et al. (2018) determined that the O-isotopic composition and Al–Mg age data are reasonably consistent among several different achondrites: the ungrouped NWA 11119 and NWA 7325, and the trachyandesite clasts in the Almahatta Sitta anomalous polymict ureilite (MS-MU-011/ALM-A and MS-MU-035). In addition, some notable geochemical similarities have been identified. Moreover, it was recognized that these are the only achondrites known that plot on the carbonaceous chondrite anhydrous mineral (CCAM) line at the high-δ18O end. Although these meteorites do have distinct Fe/Mn ratios, the range in values is much less than that within the acapulcoite–lodranite clan, the latter of which is known to represent a single parent body. The many similarities that have been documented among these diverse meteorites suggest that a genetic relationship (i.e., a common differentiated parent body) may exist (see diagram below). Oxygen Three-Isotope Plot
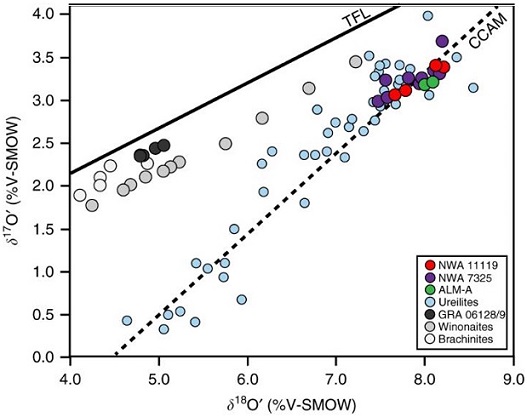
Diagram credit: Srinivasan et al., Nature Communications, vol. 9, p. 4 (2018, open access link)
‘Silica-rich volcanism in the early solar system dated at 4.565 Ga’
(https://doi.org/10.1038/s41467-018-05501-0)
For additional information on the NWA 11119 meteorite, read the PSRD article by G. Jeffrey Taylor—The Oldest Volcanic Meteorite: A Silica-Rich Lava on a Geologically Complex Planetesimal, Aug 2018.
It is noteworthy that Vaci et al. (2018) recognized another ungrouped achondrite, NWA 5517, that might plot along an extension of the O-isotopic trend line for NWA 7325, NWA 11119, and the trachyandesite clast ALM-A (not plotted below). This meteorite has a poikiloblastic texture composed of 56 vol% equigranular olivine, 42 vol% clinopyroxene (Cr-diopside) oikocrysts enclosing smaller olivines, and 2 vol% FeNi-metal and troilite (Bunch and Wittke, NAU; D. Rumble III, CIW). Northwest Africa 5517 is a sub-TFL achondrite that is not derived from the carbonaceous chondrite reservoir, and it could possibly represent a mafic relative of the other two intermediate meteorites. sub-TFL Achondrites
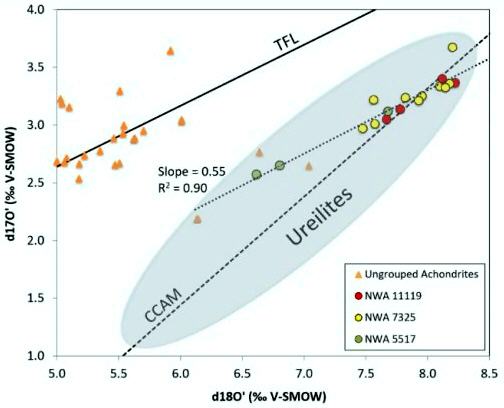
Diagram credit: Vaci et al., 49th LPSC, #1256 (2018) Early in solar system history a number of planestesimals grew to protoplanetary and even planetary sizes, underwent gravitational differentiation, and developed a rotating core dynamo that produced a weak magnetic field. Evidence for this is found in the paleomagnetic signatures detectable today in their associated achondritic meteorites. The strength of the natural remanent magnetization of the NWA 7325 meteorite was measured in paleomagnetic studies conducted by Weiss et al. (2013, 2017). While a measurable paleomagnetic signature has been detected for all achondrites studied to date, some reflecting the parent body surface magnetic fields at the time of their formation measuring hundreds of microteslas (µT = unit of magnetic field strength), the magnetic paleointensity of NWA 7325 at the time of cooling was found to be negligible—less than ~1.7 µT. This is comparable to the magnetic field intensity on the surface of Mercury as measured by the MESSENGER spacecraft.
 |
This diagram illustrates the comparative scale of the differentiated layers of Mercury |
A methodical and systematic analysis of the delivery criteria for a mercurian meteorite was presented by W. Vaughan and J. Head (2014), with reference to current improved data obtained by the MESSENGER spacecraft. In lieu of providing a synopsis of their conclusions here, their concise LPSC abstract is best read in its entirity. Although the transfer dynamics of ejectaFractured and/or molten rocky debris thrown out of a crater during a meteorite impact event, or, alternatively, material, including ash, lapilli, and bombs, erupted from a volcano. Click on Term to Read More from Mercury to Earth are more complex than delivery from Mars, the probability of finding a Mercurian meteorite may be higher than once thought. Since the acquisition of geochemical data from orbitThe elliptical path of one body around another, typically the path of a small body around a much larger body. However, depending on the mass distribution of the objects, they may rotate around an empty spot in space • The Moon orbits around the Earth. • The Earth orbits around Click on Term to Read More is significantly affected by the high surface temperatures on Mercury, verification of a mercurian origin for NWA 7325 must await better methods to obtain ground truth.
In a noble gas study of NWA 7325 conducted by Hopp et al. (2018), a shielding analysis was used to infer a relatively small pre-atmospheric radius for the parent meteoroidSmall rocky or metallic object in orbit around the Sun (or another star). of NWA 7325 of a few decimeters (1 ft = 3.048 dm). For further information about the criteria for identifying a mercurian meteorite, visit the Indicators of a Mercurian Meteorite page on this website. The specimen of NWA 7325 shown above is a 0.379 g cut fragment. The photo collage below shows some of the stones obtained by Stefan Ralew which were classified as NWA 7325. NWA 7325
click on image for a magnified view
Courtesy of Stefan Ralew—SR-Meteorites







