Angra dos Reis
AngriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More
Pyroxenite
PlutonicGeology: Igneous intrusive body that forms when magma is injected into host rocks and solidifies. Plutons occur in the crust of asteroids undergoing differentiation or planets. Named after Pluto, the Roman god of the underworld. Plutonic rocks are the rocks found within a pluton. Astronomy: Category of planet including all Click on Term to Read More/Metamorphic 

click on photos for a magnified view Fell January 20, 1869
22° 58′ S., 44° 19′ W. At 5:00 A.M. in Rio de Janeiro, Brazil, a stone weighing ~1.5 kg was seen to fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More. The meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More left a smoke trail as it plunged into the bay to a depth of about 2 meters. Two small pieces were recovered by a diver the following day. An unmatched fresh surface on one of the fragments indicates that a third piece was not recovered. One fragment was described at the time as weighing 444.5 g, but no reference was made to the other piece. Unfortunately, there is only ~150 g of Angra dos Reis (AdoR) accounted for in collections today. More than one hundred years passed since the fall and classification of Angra dos Reis until other angrites were found, primarily in the cold and hot deserts of the world. The relatively small number of unique angrites represented in our collections today have been grouped as basaltic/quenched, sub-volcanic/metamorphic, and plutonic/metamorphic, along with a single dunitic sample in NWA 8535 (photo courtesy of Habib Naji). Angra dos Reis is the only pyroxenite among the known angrites, composed of 93 vol% clinopyroxene in the rare form of Al,Ti–diopside-hedenbergite, formerly known as fassaite. This fassaite is present in two textures: 1) poikilitic megacrysts up to 3 mm in size, possibly representing phenocrysts or relict cumulus grains, and 2) groundmass grains ~100 µm in size, possibly derived from devitrified melt or by an annealing process (Treiman, 2011). Historically, Angra dos Reis had been thought to have crystallized as a cumulateIgneous rock composed of crystals that have grown and accumulated (often by gravitational settling) in a cooling magma chamber. Click on Term to Read More, or possibly from a fractionated melt, but uniquely, it contains minor calcic ferroan olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More incorporating magnesian kirschsteinite which exsolved from the olivine during slow cooling or annealing (Fittipaldo et al., 2003, 2005). Kirschsteinite also occurs between grains in olivine aggregates, often associated with troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More, which suggests an origin from a melt residue. Rare kirschsteinite lamellae also occur within some olivine grains in olivine aggregates. The Al,Ti–diopside-hedenbergite, olivine, and kirschsteinite mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More components each have homogeneous major, minor, and trace elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More compositions consistent with extended equilibration. Based on studies of how kirschsteinite lamellae profiles relate to cooling rates, the burial depth of the angrites as they crystallized in a lavaHot molten or semifluid rock derived from a volcano or surface fissure from a differentiated and magmatically active parent body. Click on Term to Read More field is inferred to have been 15–75 m; by comparison, the quench-textured angrites (e.g., D’Orbigny and Sahara 99555) could have crystallized within a meter of the surface. Minor constituents of AdoR include FeNi-metal, spinelMg-Al oxide, MgAl2O4, found in CAIs., and whitlockite, along with rare Ti–magnetiteFe oxide, FeFe2O4, containing oxidized iron (Fe) found in the matrix of carbonaceous chondrites and as diagnostic component in CK chondrites. In CK chondrites, magnetite is typically chromian, containing several wt. % Cr2O3. Click on Term to Read More, plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More, celsian, and baddelyite. Angra dos Reis is highly depleted in volatiles such as Na, and highly enriched in oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More elements such as FeO, TiO and CaO, characteristics which distinguish this meteorite from those of other groups. The angriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More source region can be modelled as an incomplete mixing of an alkali- and metal-depleted primitive chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More with high-Ca, high-temperature condensates similar to CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More, but containing excess meliliteGroup of minerals found in the CAIs of meteorites such as CV chondrites. Melilite consists almost exclusively of the binary solid solution gehlenite (Ca2Al2SiO7) – åkermanite (Ca2MgSi2O7). The melilite in CAIs is closer to gehlenite in composition. The first-formed (highest-temperature) melilite crystallizing from a melt is relatively aluminum-rich and becomes progressively Click on Term to Read More. Angra dos Reis is an extremely ancient basaltic meteorite, and extensive isotopic studies have established that it is an early planetary differentiate undisturbed since its crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More ~4.5566 b.y. ago. Among the limited suite of angrites, it remains the best representative of the original mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More isotopic composition (Abernethy et al., 2013). Another group of angrites have isochrons reflecting a more rapid cooling history, crystallizing up to 7 m.y. earlier than AdoR. The relatively late crystallized AdoR has a minor δ26Mg content that might reflect the Mg isotopic composition of the APB after 26Al decay (Schiller et al., 2010). Two radically divergent models for the formation of the angrites have been presented. One was proposed by Kurat et al. (2004), a brief synopsis of which can be found on the D’Orbigny page. They present evidence for a non-igneous origin of angrites on a very early-formed parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More which was composed primarily of refractory material. Another scenario was proposed by King and Henley (2016), in which angrites formed within a small dust/gas clump that existed in the protoplanetary discFlattened and rotating disk of dense gas and dust/solids orbiting a young star from which planets can eventually form. Click on Term to Read More, rather than on a large differentiated object. The decay products of extinct radionuclides in AdoR suggest that the entire sequence from nebular condensation through parent body accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More, partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More of the parent body, metallic coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More formation, formation of clinopyroxene rock, cumulate/crystallization processes, and final cooling to temperatures low enough to retain fissionBreaking apart of a body into smaller fragments. In nuclear physics, fission refers to splitting of a heavy atomic nucleus into two or more lighter nuclei with an associated release of energy. The mass of the nucleus before fission is greater than the combined masses of the resulting fragments; the Click on Term to Read More tracks and noble gasesElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More took an incredibly short 18 m.y. Crystallization of AdoR proceeded as a two-stage process beginning with partial melting from a source composed of olivine, orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg substitutes for Fe up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca contents occur Click on Term to Read More, and clinopyroxene at low pressure, followed by an extended period of slow cooling and annealing to ~650°C, after which time it was rapidly quenched during a severe impact event. Vigorous outgassing, element fractionationConcentration or separation of one mineral, element, or isotope from an initially homogeneous system. Fractionation can occur as a mass-dependent or mass-independent process. Click on Term to Read More (e.g., Si), and evaporationProcess in which atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to enter the gaseous state. Click on Term to Read More of volatiles likely occurred during the planetesimal accretionary stage (Pringle et al., 2015), as well as during severe impact events; impact-induced devolatilization was possibly hastened by the reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More strength of the gravitational field following asteroid fragmentation. Despite the extreme volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). depletions in angrites, a high water content has been measured in silicates (20–60 ppmParts per million (10). Click on Term to Read More) and phosphates (>400 ppm) in both AdoR and D’Orbigny, and this water is highly enriched in deuteriumAlso called heavy hydrogen, deuterium is an isotope of hydrogen (D, or H) whose nucleus contains one proton and one neutron. As a trace element formed during the nucleosynthesis epoch of the Big Bang, deuterium is an important indicator of the baryon density in the universe. The larger the density, the Click on Term to Read More (δD >500‰) compared to the Earth (Sarafian et al., 2015). The volatile-depleted nature of angrites may reflect accretion from volatile-poor precursor material, followed by early accretion of either D-rich water or water that subsequently experienced strong degassing and fractionation; however, other scenarios are also possible. In contrast to the unshocked, unbrecciated nature of other angrites, Angra dos Reis is an unbrecciated meteorite that has experienced a significant shock event or thermal metamorphism. Scott and Bottke (2011) proposed that the unshocked appearance of AdoR is most consistent with a long-term storage residence of several b.y. following a catastrophic impact ~4.5 b.y. ago. This storage period commenced after angrite material was ejected and accreted into one or more small, secondary angritic bodies ~10 km in diameter. They reason that an original parent body <200 km in diameter would have resulted in a loss of basalts through explosive volcanism, and that the presence of trapped solar-type gases, presence of possible high-pressure intergrowth phases, and evidence of an ancient core dynamo, are factors consistent with a large parent body. Paleomagnetic intensity studies conducted for Angra dos Reis by Wang et al. (2015) have established a natural remanent magnetization value of ~15 µT (microTeslas), demonstrating that this lithology formed under the influence of a significant core dynamo which existed ~11 m.y. after CAIs. By comparison, no natural remanent magnetization (paleointensity) > ~1 µT was detected for the earlier formed angrites D’Orbigny and Sahara 99555, which constrains the onset of the APB core dynamo to later than ~4 m.y. after CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More formation. It was also recognized that the strong solar nebula-generated magnetic field which had existed ~1.2–3 m.y. after CAIs (~50 µT, measured in Semarkona chondrulesRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More) had virtually disappeared by the time the earliest angrites were formed, indicating that the solar nebulaThe primitive gas and dust cloud around the Sun from which planetary materials formed. had already been largely dissipated.
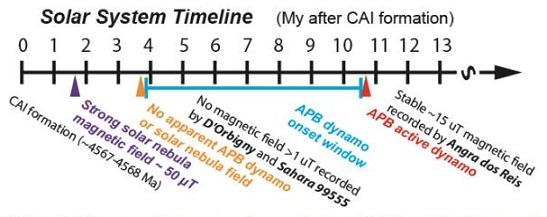
Diagram credit: Wang et al., 46th LPSC, #2516 (2015) Since Angra dos Reis is anomalous in its mineralogy and has aberrant major and trace elemental compositions compared to other angrites, it has been proposed that AdoR represents either a separate source magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More on the angrite parent body that experienced a unique thermal history, or that it represents an entirely distinct parent body. It was concluded by Kleine et al. (2009) that both AdoR and the plutonic/metamorphic angrite NWA 2999 were derived from a parental source magma which had higher Hf/W than other angrites, likely the result of extended differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More after core formation. Recent investigations by Tonui et al. (2003) into the initial 87Sr/86Sr in Angra dos Reis and D’Orbigny have determined that their parent sources were similar, and they have provided actual evidence that AdoR and D’Orbigny, and probably the other angrites, share a common parent body. Moreover, O-isotope analyses conducted for AdoR and several other angrites clearly indicate that all angrites studied originated from a single parent body (Greenwood et al., 2003). This O-isotope study also included diverse members of the HED suite (thought to originate on the asteroid 4 VestaThird largest and fourth brightest asteroid; it was discovered in 1807 by Heinrich Olbers and named for the ancient Roman goddess of the hearth. 4 Vesta has a basaltic surface composition and an average density not much less than that of Mars. Evidently lava once flowed here indicating that the), and it was concluded that HED meteorites represent a single parent body unique from the angrite parent body. The Rb–Sr chronometry of angrites as it relates to CAIs indicates that a possible late volatile depletion occurred, which is difficult to reconcile with very early accretion and differentiation (Hans et al., 2010). The similarity in Δ17O values between angrites and the ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More iron Tishomingo (based on anaysis of a stishoviteDense, high-pressure phase of quartz; so far identified only in shock-metamorphosed, quartz-bearing rocks from meteorite impact craters. Stishovite was synthesized in 1961 before it was discovered at Meteor Crater, Arizona. Its structure consists of parallel chains of single SiO6 octahedra. The octahedra are on their sides, sharing opposing edges. Image Click on Term to Read More grain) suggests that a genetic relationship might exist (Corrigan et al., 2005, 2017 [see diagram below]). Furthermore, both angrites and Tishomingo formed from a volatile-depleted precursor under oxidizingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions. Investigators have also explored the possibility of a genetic relationship between angrites and IVB irons (e.g., Campbell and Humayun, 2005), as well as between Tishomingo and IVB irons (e.g., Corrigan et al., 2005). Based on O-isotopic analyses utilizing chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More grains from IVB irons Warburton Range and Hoba, Corrigan et al. (2017) concluded that IVB irons are not genetically related to either angrites or to Tishomingo, but that their respective parent bodies experienced similar petrogenetic histories. Beyond that, Burkhardt et al. (2011) determined that differences in both O- and Mo-isotopic compositions between angrites and IVB irons exclude a genetic linkage.

Diagram credit: Corrigan et al., 48h LPSC, #2556 (2017) Utilizing stepped combustion analyses to study the indigenous carbonElement commonly found in meteorites, it occurs in several structural forms (polymorphs). All polymorphs are shown to the left with * indicating that it been found in meteorites and impact structures: a. diamond*; b. graphite*; c. lonsdalite*; d. buckminsterfullerene* (C60); e. C540; f. C70; g. amorphous carbon; h. carbon nanotube*. Click on Term to Read More and nitrogenPrincipal constituent of the Earth’s atmosphere (78.08 vol. % at ground level). Nitrogen is the fifth most abundant element in the universe by atom abundance. Nitrogen comprises only 3.5 vol. % of the atmosphere of Venus and 2.7 vol. % of Mars’s atmosphere. Nitrogen has two isotopes: N (99.632 %) and N Click on Term to Read More component of five of the angrites, including Angra dos Reis, Abernethy et al. (2013) found that both of these light elements were released at similar temperatures (700–1200°C). Although they could not determine a specific correlation between the two elements based on their abundances or their isotopic compositions, the team did demonstrate the likelihood that much of the C and N was incorporated as atoms within the silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the latticeRegularly spaced array of points that represents the structure of a crystal. Crystals are composed of groups of atoms repeated at regular interval in three dimensions with the same orientation. The smallest division of the lattice which can still be used to represent the entire structure is called the unit Click on Term to Read More, probably attained through metasomatic processes involving sulfur-rich fluids. It was further hypothesized that the atomic C originated from graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More, itself being an earlier product of a carbonateMineral or compound containing carbon and oxygen (i.e. calcium carbonate, CaCO3, calcite). Click on Term to Read More reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More process, or that it was a result of dissociation of CO2. In a similar manner, it was shown that atomic N was likely dissociated at high temperatures and then became bound within the silicate lattice. There remains a speculation at this point that some of the C and/or N was originally an organicPertaining to C-containing compounds. Organic compounds can be formed by both biological and non-biological (abiotic) processes. Click on Term to Read More component of a carbonaceous phase similar to that found in CM-type carbonaceous chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More. It was inferred by Nyquist and Bogard (2003) that since the angrite D’Orbigny was spectroscopically similar to two asteroids located ~2.8–2.9 AUThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More (289 Nenetta and 3819 Robinson), then it was also probable that the angrite parent body formed in this same region. They argued that asteroids at this heliocentricCentered around a sun. Our own Solar System is centered around the Sun so that all planets such as Earth orbit around the Sun. Note that 25% of Americans incorrectly believe the Sun revolves around the Earth. Click on Term to Read More distance accreted too slowly to permit the accumulation of enough 26Al to cause global melting and differentiation before a diameter greater than ~200 km would have been attained; i.e., given a body with a diameter larger than ~200 km, there would not have been enough heat necessary to melt and differentiate this body. By this line of reasoning, it may be concluded that the differentiated angrite parent body was either not as large as 200 km in diameter, or that it formed at a smaller heliocentric distance than ~2.8 AU. Without regard to heliocentric distance, Sanders and Scott (2007) argued that any body which accreted to a diameter >60 km (i.e., large enough to minimize heat loss from the surface through conductionTransfer of heat as a result of collisions between molecules; when one end of an object is heated or excited, the molecules vibrate faster and their energy is transferred sequentially to their neighbors. Click on Term to Read More) within ~2 m.y. of CAI formation (the oldest objects dating to 4.567 b.y. ago) as the angrites did, must contain enough 26Al to produce global melting and differentiation. In contrast, Senshu and Matsui (2007) determined that accretion to a diameter of only ~14 km occurring within 2 m.y. of CAI formation was all that was required for global differentiation to occur, while a diameter of 40–160 km occurring within 1.5 m.y. was cited by Hevey and Sanders (2006) and Sanders and Taylor (2005) as the minimums. Be that as it may, John T. Wasson (2016) presented evidence that the slow heating generated entirely by the decay of 26Al is insufficient to melt asteroids, and that an additional heat source would have been required; e.g., the rapid heating incurred from major impact events. He determined that the canonical 26Al/27Al ratio of 0.000052 is much too low to cause any significant melting, and that a minimum ratio of 0.00001 would be required to produce a 20% melt fraction on a well-insulated body having a significant concentration of 26Al. For example, the initial ratio of 0.0000004–0.0000005 calculated for the angrites Sah 99555 and D’Orbigny based on their 26Al–26Mg isochrons is too low to have generated any significant melting without an additional heat source. Therefore, impacts were a major source of heating in early Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. history. It has also been suggested by some that relatively small planetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More might have been just the required size to allow heating by induction in the plasmaFourth state of matter: a gas in which many or most of the atoms are ionized. In the plasma state the atoms have split into positive ions and negative electrons, which can flow freely, so the gas becomes electrically conducting and a current can flow. Click on Term to Read More environment of the T Tauri SunOur parent star. The structure of Sun's interior is the result of the hydrostatic equilibrium between gravity and the pressure of the gas. The interior consists of three shells: the core, radiative region, and convective region. Image source: http://eclipse99.nasa.gov/pages/SunActiv.html. The core is the hot, dense central region in which the. In order to better constrain the properties of the differentiated angrite parent body core, van Westrenen et al. (2016) conducted a study modeling siderophile elementLiterally, "iron-loving" element that tends to be concentrated in Fe-Ni metal rather than in silicate; these are Fe, Co, Ni, Mo, Re, Au, and PGE. These elements are relatively common in undifferentiated meteorites, and, in differentiated asteroids and planets, are found in the metal-rich cores and, consequently, extremely rare on depletions along with their metal–silicate partitioningThe tendency of elements to prefer one mineral phase relative to another or to preferentially enter the solid or remain in the liquid during crystallization. Click on Term to Read More behavior for the hypothesized angrite parental melt composition. A CV chondrite mantle composition was used for their calculations, along with a temperature and pressure (0.1 GPa) appropriate for a solidifying melt on a small planetesimal. Their results indicate that the observed siderophile element depletions of angrites are consistent with a core mass fraction of 0.12–0.29 composed of Fe and Ni in a ratio of ~80:20 (with a low S content), and that it was formed under redoxOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions (oxygen fugacityUsed to express the idealized partial pressure of a gas, in this case oxygen, in a nonideal mixture. Oxygen fugacity (ƒO2) is a measure of the partial pressure of gaseous oxygen that is available to react in a particular environment (e.g. protoplanetary disk, Earth's magma, an asteroid's regolith, etc.) and Click on Term to Read More) of ΔIW–1.5 (±0.45). Precise U–Pb ages have been calculated for AdoR and LEW 86010 to be 4.55765 (±0.00013) b.y. and 4.55855 (±0.00015) b.y., respectively (Y. Amelin, 2007). An identical age within error, based on Mn–Cr systematics, was established for the angrite NWA 2999—it was determined to be 4.5579 (±0.0011) b.y. old. Although these three angrites are slowly-cooled basalt-type rocks exhibiting unzoned minerals, they crystallized over an extended period of at least 0.90 (±0.19) m.y., and therefore, were likely derived from independent magma sources. Based on a comparison of Hf/Sm ratios for a diverse sampling of both angrites and eucritesMost common type of achondrite meteorite and a member of the HED group. Eucrites are basalts composed primarily of pigeonite and anorthite (An60-98). Eucrites have been placed into three subgroups based on mineralogical and chemical differences. • Non-cumulate eucrites represent the upper crust that solidified on a magma ocean after Click on Term to Read More, Bouvier et al. (2015) inferred that these two meteorite groups reflect the existence of three distinct crustal reservoirs on their respective parent bodies. These three reservoirs reflect similar chemical differentiation processes on both parent bodies: 1) subchondritic Hf/Sm ratios for the Angra dos Reis angrite and the cumulate eucrites (such as Moama); 2) chondritic Hf/Sm ratios for the quenched angrites (such as D’Orbigny and Sahara 99555) and the basaltic eucrites; 3) superchondritic Hf/Sm ratios for the plutonic angrites (NWA 4590 and NWA 4801) and the unusual cumulate eucriteMost common type of achondrite meteorite and a member of the HED group. Eucrites are basalts composed primarily of pigeonite and anorthite (An60-98). Eucrites have been placed into three subgroups based on mineralogical and chemical differences. • Non-cumulate eucrites represent the upper crust that solidified on a magma ocean after Click on Term to Read More Binda. Cosmic-ray track densitiesMass of an object divided by its volume. Density is a characteristic property of a substance (rock vs. ice, e.g.). Some substances (like gases) are easily compressible and have different densities depending on how much pressure is exerted upon them. The Sun is composed of compressible gases and is much Click on Term to Read More place the pre-atmospheric mass of AdoR at ~80 kg, with an exposure ageTime interval that a meteoroid was an independent body in space. In other words, the time between when a meteoroid was broken off its parent body and its arrival on Earth as a meteorite - also known simply as the "exposure age." It can be estimated from the observed effects Click on Term to Read More of 55.5 (±1.2) m.y. (Lugmair and Marti, 1977). Multiple episodes of impact, disruption, and dissemination of the crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More can be inferred by the wide range of CRE ages determined for the angrites—<0.2–56 m.y. for thirteen angrites measured to date, possibly representing as many ejection events (Nakashima et al., 2008; Wieler et al., 2016; Nakashima et al., 2018). This range is consistent with a single large parent body enduring multiple impacts over a very long period of time, which would suggest that the parent object resides in a stable orbitThe elliptical path of one body around another, typically the path of a small body around a much larger body. However, depending on the mass distribution of the objects, they may rotate around an empty spot in space • The Moon orbits around the Earth. • The Earth orbits around Click on Term to Read More (planetary or asteroid beltBelt located between 2.12 and 3.3 AU from the Sun and located between the orbits of Mars and Jupiter containing the vast majority of asteroids. The asteroid belt is also termed the main asteroid belt or main belt to distinguish it from other asteroid populations in the Solar System such Click on Term to Read More) permitting continuous sampling over at least the past 56 m.y. Alternatively, Nakashima et al. (2018) consider it plausible that there is currently at least two angrite (daughter) objects occupying distinct orbits: one representing the fine-grained (quenched) angrites with the shorter CRE age range of <0.2–22 m.y., and another representing the coarse-grained (plutonic) angrites with the longer CRE age range of 18–56 m.y. (see diagram below). Cosmic-ray Exposure Ages of Angrites
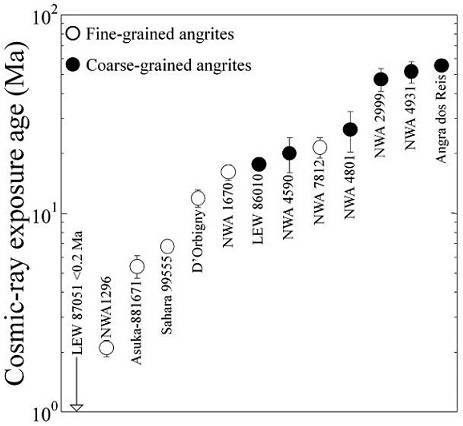
Diagram credit: Nakashima et al., MAPS, Early View, p. 14 (2018)
‘Noble gases in angrites Northwest Africa 1296, 2999/4931, 4590, and 4801: Evolution history inferred from noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More signatures’
(http://dx.doi.org/10.1111/maps.13039) Notably, Rivkin et al. (2007) have determined that the largest known co-orbiting ‘Trojan’ asteroid of Mars, the 1.3 km-diameter 5261 Eureka located at a trailing Lagrangian point, is a potentially good spectral analog to the angrites (as measured by Burbine et al., 2006) (see diagrams below). They suggest that 5261 Eureka could represent a captured fragment of the disrupted angrite parent body now in a stable orbit around Mars.
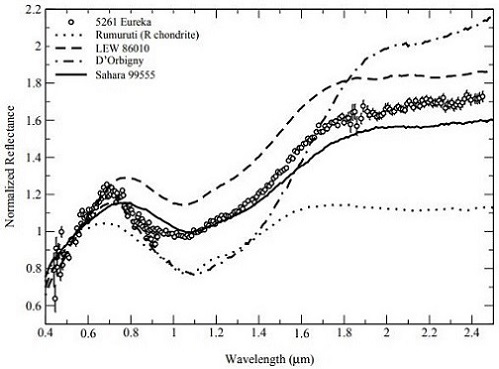
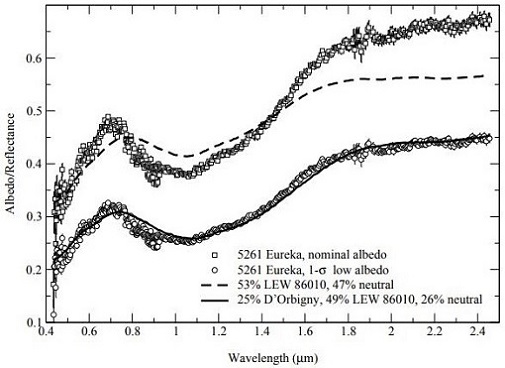
Diagrams credit: Rivkin et al., Icarus, vol. 192, #2, (2007)
‘Composition of the L5 Mars Trojans: Neighbors, not siblings’
(https://doi.org/10.1016/j.icarus.2007.06.026; open access link) The photos of AdoR shown above depict both sides of a 0.34 g fragment with a small patch of glossy black fusion crustMelted exterior of a meteorite that forms when it passes through Earth’s atmosphere. Friction with the air will raise a meteorite’s surface temperature upwards of 4800 K (8180 °F) and will melt (ablate) the surface minerals and flow backwards over the surface as shown in the Lafayette meteorite photograph below. Click on Term to Read More visible in the top picture (click on the photos for a magnified view). A photo of the main massLargest fragment of a meteorite, typically at the time of recovery. Meteorites are commonly cut, sliced or sometimes broken thus reducing the size of the main mass and the resulting largest specimen is called the "largest known mass". Click on Term to Read More of Angra dos Reis, curated at the National Museum of Brazil, is shown below courtesy of Andre Moutinho. This photo exhibits clearly the extensive area of rippled, glossy black fusionProcess in which two lighter atomic nuclei combine to form a heavier atomic nucleus. Very high temperatures are normally required in order for atomic nuclei to collide with sufficient energy to overcome the Coulomb barrier (their mutual electrostatic repulsions). Fusion that occurs under high-temperature conditions is called thermonuclear fusion. Fusion Click on Term to Read More crust.

click on photo for a magnified view Tragedy struck the 200-year-old National Museum of Brazil in Rio de Janeiro during the evening of September 2, 2018, when a fire quickly spread through the structure. According to curator María Elizabeth Zucolotto, 30 out of 33 meteorites that were on display have been recovered, and the 70 g main mass of Angra dos Reis was eventually recovered from the office safe. The angrite meteorite is reported to have an estimated value of approximately $750,000.



Photos courtesy of National Museum of Brazil






