Moss
CO3.6
Fell July 14, 2006
59° 27′ N., 10° 42′ E.
On Friday, July 14 at 10:15 A.M., a fireballA fireball is another term for a very bright meteor, generally brighter than magnitude -4, which is about the same magnitude of the planet Venus as seen in the morning or evening sky. A bolide is a special type of fireball which explodes in a bright terminal flash at its end, often with visible fragmentation. Click on Term to Read More traveling in a north-northwest direction exploded over the town of Moss, Norway, in the county of Ostfold, located about 50 km south of Oslo on the east side of Oslofjord. The fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More was accompanied by a loud explosion and thunderous rumblings, and numerous calls and reports were made by eyewitnesses to the event. A clear signal was picked up at the Norwegian Seismic Array (NORSAR) in Kjeller, Norway.
MeteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More fragments fell over a wide area. In the vicinity of Rygge, located ~6 km southeast of Moss, the sounds were heard by Ragnar Martinsen as he occupied the outhouse behind his holiday cabin. As he exited, he heard a whistling sound followed by the clatter made by a 36.7 g stone hitting a corrugated metal sheet lying just 2 m away. He contacted astronomers from the Astrophysics Institute of the University of Oslo and from the Norwegian Astronomical Society and invited them to his cabin for their educated opinions of his find—they agreed it was a chondritic meteorite. On Monday, July 17, with intentions of mowing his lawn, Frode Johansen of Moss, Norway discovered a 752 g stone lodged in a 7 cm-deep hole beneath his plum tree; three broken branches attest to its flight path. The family expressed their intensions to donate the meteorite to the Museum of Natural History in Oslo. On Wednesday, July 19, a large, partially-crusted fragment was found by a local resident northwest of the Johansen findMeteorite not seen to fall, but recovered at some later date. For example, many finds from Antarctica fell 10,000 to 700,000 years ago. Click on Term to Read More. After reading about the search for the meteorite in the newspaper, he contacted meteorite hunters Michael Mazur and Bjorn Sorheim of Norway. On Sunday, July 23, the resident met with them to show them a fragment of the fall and to share details of the find location. Subsequently, Michael and Bjorn recovered additional fragments from the original 1.5 kg stone that had shattered upon impact with a fence. On Sunday evening, July 30, Morten Bilet and Michael Farmer were searching near Moss for additional pieces from this fall. They found ~800 g of fragments that had been strewn about following the impact of a single stone on concrete. Some fragments were found in pristine condition up to 20 m away from the point of impact. On Friday, August 4, a 676 g stone was found which had fallen part way through the roof of a storage warehouse belonging to the Norgesgruppen business in Moss. It had punched a 10-cm hole through the roofing material and was lodged within. Its discovery was made only after rainwater leaked through the hole that it had created. This particular stone is a natural fit to the Johansen stone, and likewise has been donated to the Museum of Natural History in Oslo. Portions of the preceeding accounts were gleaned from the Aftenposten: News From Norway (http://www.aftenposten.no/english).
Moss is the sixth witnessed fall of a CO3 chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, the first since Kainsez fell in Russia in 1937. Significantly more material was recovered from Kainsez than from Moss—200 kg compared to 3.76 kg, respectively. While the chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More content of fayalitic olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More can provide petrologic typeMeasure of the degree of aqueous alteration (Types 1 and 2) and thermal metamorphism (Types 3-6) experienced by a chondritic meteorite. Type 3 chondrites are further subdivided into 3.0 through 3.9 subtypes. calibration between 3.0 and 3.1, the low Cr value measured in Moss indicates it is higher than 3.1. A petrographic analysis of Moss was conducted by J. Grossman (US Geological Survey, Reston, VA.), which was based on the technique employed by Chizmadia et al. (2002). This analysis suggests a classification between CO3.5 and CO3.6, and Moss is listed as a CO3.6 in MetBull 91. Petrographic studies by other investigators have provided indications of a lower type (Greenwood et al., 2007). According to the Fe–Mg zoning profiles of olivine in type-I chondrulesRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More, a petrologic type closer to 3.4/3.5 was indicated.
Moss contains abundant sub-mm-sized chondrules typical for the CO group, which are embedded in a fine-grained gray matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More. Extensive low-temperature alteration has likely occurred within the solar nebulaThe primitive gas and dust cloud around the Sun from which planetary materials formed., perhaps augmented on the parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More as evidenced by wide variation in secondary transformation effects in individual inclusions. Olivine grains within AOAs and spinelMg-Al oxide, MgAl2O4, found in CAIs. within CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More have been altered to a range of higher FeO levels, while alteration of CAIs has resulted in the production of secondary mineralsMineral that forms through processes such as weathering, and in the case of meteorites can also include pre-terrestrial alteration. Secondary minerals in meteorites that formed during terrestrial weathering include oxides and hydroxides formed directly from metallic Fe-Ni by oxidation, phosphates formed by the alteration of schreibersite, and sulfates formed by Click on Term to Read More such as nepheline replacing primary meliliteGroup of minerals found in the CAIs of meteorites such as CV chondrites. Melilite consists almost exclusively of the binary solid solution gehlenite (Ca2Al2SiO7) – åkermanite (Ca2MgSi2O7). The melilite in CAIs is closer to gehlenite in composition. The first-formed (highest-temperature) melilite crystallizing from a melt is relatively aluminum-rich and becomes progressively Click on Term to Read More and/or anorthiteRare compositional variety of plagioclase and the calcium end-member of the plagioclase feldspar mineral series with the formula CaAl2Si2O8. Anorthite is found in mafic igneous rocks such as anorthosite. Anorthite is abundant on the Moon and in lunar meteorites. However, anorthite is very rare on Earth since it weathers rapidly Click on Term to Read More, and perovskiteTerm applied to A2+B4+O3 high-pressure minerals with a perovskite structure (general formula ABX3) where "A" is a metal that forms large cations such as Mg, Fe or Ca, "B" is another metal that forms smaller cations such as Si (called silicate perovskite), Ti and to a lesser degree Al, and Click on Term to Read More that has been converted to ilmeniteTi-Fe oxide, TiFeO3, found in achondrites, lunar mare basalts, and shergottites. Ilmenite forms as a primary mineral in mafic igneous rocks. It crystallizes relatively early out of a magma before most of the other minerals, and as a result, the heavier crystals of ilmenite precipitate to the bottom of the magma Click on Term to Read More (Bischoff and Schmale, 2007).
Based on 3He and 21Ne values, the CRE ages of the various CO3 chondrite falls show a range of 3.5–57 m.y., each representing an independent ejection event except for the 21 m.y. ages shared by Kainsaz and Ornans (Bartoschewitz et al., 2010). Cosmic-ray exposure values for Moss are the second shortest known for CO chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More at 14 m.y. A gas retention age of 3.95 b.y. was found, while a K–Ar age of 4.43 b.y. was found; the reason for this age difference in Moss has not yet been determined. A study of the trapped noble gasesElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More shows that Q-Xe compositions are dominant, with small contributions from air-Xe and possibly from Xe-HL (a mixture of Xe-H [enriched in heavy xenon isotopes] and Xe-L [enriched in light xenon isotopes]; Bekaert et al., 2018 and references therein). It was also shown that Ar and Kr are present in higher abundances than normal for Q-type gases, which is likely attributable to an additional carrier phase (see the Yilmia page for further details about Q-gases). No correlation was found to exist between trapped noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More abundances and metamorphicRocks that have recrystallized in a solid state due to changes in temperature, pressure, and chemical environment. Click on Term to Read More grade.
The volume of FeNi-metal and FeS is typical for the proposed classification. As with the CO3.3 Ornans and CO3.1 Kainsaz, Moss contains a low C abundance (0.25 wt%) with a heterogeneous distribution of organicPertaining to C-containing compounds. Organic compounds can be formed by both biological and non-biological (abiotic) processes. Click on Term to Read More species, all of which have a low molecular weight with a low degree of variation (Pearson et al., 2007). This C abundance is unexpectedly low given its relatively low metamorphic grade, and low when compared to the organic inventory of analogous CO3 members such as CO3.5 Lancé. A micro-Raman study of the organic matter in Moss was undertaken by Yesiltas et al. (2016), and their results indicate the presence of more ordered carbonElement commonly found in meteorites, it occurs in several structural forms (polymorphs). All polymorphs are shown to the left with * indicating that it been found in meteorites and impact structures: a. diamond*; b. graphite*; c. lonsdalite*; d. buckminsterfullerene* (C60); e. C540; f. C70; g. amorphous carbon; h. carbon nanotube*. Click on Term to Read More in some regions of the meteorite consistent with a higher degree of thermal metamorphism than in other CO chondrites. Ornans, Kainsaz, and Moss appear to have experienced unique metamorphic conditions on the parent body.
An O-isotopic analysis of Moss conducted by Franchi and Greenwood (The Open University, UK) provided values which are consistent with the CO carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More group. A subsequent precise O-isotopic investigation was conducted by Greenwood et al. (2016) of a broad sampling of CO chondrites, including six CO falls (Moss, Feliz, Kainsaz, Lancé, Ornans, and Warrenton) and fourteen primitive Antarctic finds representing at least four distinct meteorites (e.g., DOM 08006 [3.00], DOM 08004, MIL 03377/07099, and ALH 77307 [3.03]). The resulting plots on an oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More three-isotope diagram resolve two separate clusters of CO chondrites—one which includes the primitive Antarctic finds and another which includes the more highly metamorphosed falls. It was noted that the isotopic values of the two more metamorphosed Antarctic meteorites (ALH 82101 [3.3] and MIL 090785 [3.7]) plot within the field of the more metamorphosed CO falls, in support of the existence of two distinct clusters. The investigators suggest that these two CO clusters could reflect the existence of multiple parent bodies, or perhaps more plausible, a greater interaction of the CO falls with an 16O-poor aqueous reservoir. Interestingly, the primitive Antarctic finds plot with the anhydrous silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the component of the CM chondriteClass of carbonaceous chondrites named after the Mighei meteorite that fell in Ukraine in 1889. They represent samples of incompletely serpentinized primitive asteroids and have experience extremely complex histories. CM meteorites are generally petrologic level type 2 though a few examples of CM1 and CM1/2 also exist. Compared to CI Click on Term to Read More Murchison (see the Colony page for further information about a potential CO–CM genetic relationship). 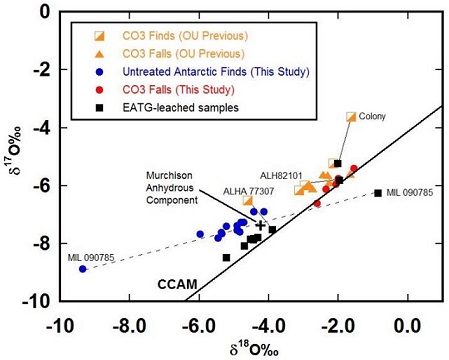
click on image for a magnified view Diagram credit: Greenwood et al., 47th LPSC, #2206 (2016) In their continued study of the CO chondrites listed above, Alexander et al. (2017) found that these meteorites could be divided into two distinct groups based on C abundances and isotopic composition. Even after accounting for isotopic variability due to terrestrial aqueous alteration, two distinct clusters are still apparent composed of the following: 1) 16O-enriched primitive Antarctic finds, and 2) 16O-poor equilibrated CO falls (and the more equilibrated ALH 82101 and Colony finds). The authors suggest the most plausible expanation for these clusters is that the more equilibrated members incorporated higher abundances of a primary 16O-poor water component compared to the primitive Antarctic finds, and they presume that a continuum would otherwise exist among all CO chondrites—bounded by an 16O-rich end member like DOM 08006 and an 16O-poor end member like the equilibrated CO falls. The specimen of Moss shown above is a 1.97 g crusted fragment. The photo below is an excellent petrographic thin sectionThin slice or rock, usually 30 µm thick. Thin sections are used to study rocks with a petrographic microscope. micrograph of Moss, shown courtesy of Peter Marmet.

click on image for a magnified view
Photo courtesy of Peter Marmet







