AngriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More
Basaltic/Quenched

Found July 1979
37° 40′ S., 61° 39′ W. This relatively fresh, 16.55 kg, shield-shaped, regmaglypted meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More is by far the largest of the angrites found so far. The mass was found in Buenos Aires Province, Argentina by a farm worker who struck it with a plow. Thinking he had unearthed an Indian artifact, possibly an old mortar, he gave it to the landowner who set it by his house for the next ~20 years. Not until 1998, after reading an article on meteorites, did the owner seek to have the stone analyzed. In September 2000, Dr. G. Kurat of the Naturhistorisches Museum in Vienna, Austria made the determination that it was an angriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More.
D’Orbigny is an unusual
achondriteAn achondrite is a type of stony meteorite whose precursor was of chondritic origin and experienced metamorphic and igneous processes. They have a planetary or differentiated asteroidal origin where the chondritic parent body reached a sufficient size that through heating due to radioactive decay of 26Al (aluminum isotope) and gravitational Click on Term to Read More that shows no evidence of
brecciationThe formation of a breccia through a process by which rock fragments of of various types are recemented or fused together. Click on Term to Read More,
shock metamorphismMetamorphism produced by hypervelocity impact between objects of substantial size moving at cosmic velocity (at least several kilometers per second). Kinetic energy is converted into seismic and heat energy almost instantaneously, yielding pressures and temperatures far in excess those in normal terrestrial metamorphism. On planetary bodies with no atmosphere, smaller Click on Term to Read More, or significant thermal metamorphism, and some believe that it might not have an igneous origin. It consists predominantly of Ca-bearing
olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More and
anorthiteRare compositional variety of plagioclase and the calcium end-member of the plagioclase feldspar mineral series with the formula CaAl2Si2O8. Anorthite is found in mafic igneous rocks such as anorthosite. Anorthite is abundant on the Moon and in lunar meteorites. However, anorthite is very rare on Earth since it weathers rapidly Click on Term to Read More, which compose an intergrowth of plates and networks (Kurat
et al., 2004). The other major constituent is Ti–Al-augite, which likely formed later than the olivine–anorthite intergrowths. A late
oxidizingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More event produced strongly zoned grains of the clinopyroxene Al,Ti–diopside-hedenbergite, previously known as fassaite, which now fill most of the intergranular spaces. Large (up to 1 cm) clear to milky, green to greenish-white, magnesian olivine megacrysts and polycrystalline olivinites represent one of the earliest phases of the host rock. Also representing very early constituents, Cr-bearing Al-spinel and Fe-bearing
spinelMg-Al oxide, MgAl2O4, found in CAIs. occur within some olivine and anorthite grains. Minor kirschsteinite, ulvöspinel, and
troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More (and other sulfides) are present, along with rare
awaruiteNi-rich Fe metal, Ni3Fe, similar to taenite found in minor amounts in some meteorites. Awaruite is also known as josephinite, a mineral found as placer deposits in Josephine County, Oregon, and sometimes mistaken for a meteorite. Note: web.mineral.com incorrectly defines Awaruite as “Ni2Fe to Ni3Fe”, however the IMA Database of Click on Term to Read More, Ca silico-apatite (a late-stage
crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More phase; Mikouchi
et al, 2010, 2015), and an unidentified Fe–Al–Ti
silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the. Rare, cm-sized, Mg- and Cr-rich olivine and spinel xenocrysts with granoblastic textures have been identified, similar to those found in greater abundance in other angrites (except Sah 99555). The xenocrysts are indicative of a rapid ascension of
magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More.
D’Orbigny has a heterogeneous composition consisting of alternating layers of a dense, coarse-grained texture, and a porous texture containing abundant round vugs or hollow shells up to 2.5 cm, along with plates and druses composed primarily of
augiteHigh-Ca clinopyroxene, (Ca,Mg,Fe)SiO3, that occurs in many igneous rocks, particularly those of basaltic composition. In order to be considered augite, the clinopyroxene must contain 20 to 45 mol % of calcium (Wo20 - 45). An important and unique Martian meteorite is NWA 8159, that has been classified as an augite basalt. Click on Term to Read More (diopside-hedenbergite) crystals and some anorthite crystals. Formation of these vesicles is consistent with bubble growth involving significant CO and CO
2 concentrations of 10–20
ppmParts per million (106). Click on Term to Read More (up to 25 ppm C) in a magma as it ascended within a
dikePlanar, blade-like, intrusive igneous body that cuts across preexisting layers usually at a high-angle to near-vertical orientation. By definition, a dike is always younger than the rocks that contain it. Terrestrial dikes are typically 0.5 to 3 m wide and extend for a few 10s of kilometers. Click on Term to Read More, undergoing decompression and coalescence of smaller bubbles, eventually solidifying near the surface (McCoy
et al., 2003, 2006). It was ascertained that the druse pyroxenes formed under oxidizing conditions (near the QFM buffer), then underwent rapid cooling from ~1000°C, perhaps as a result of the dissipation of a hot vapor (Abdu
et al., 2009).
Conversely, instead of formation of the vesicles through an igneous process, it was proposed by Kurat
et al. (2002) that they were originally solid spheres composed of one of the earliest and most
reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More phases, possibly CaS, which was covered by anorthite-olivine rims and plates. The cores were subsequently lost through an oxidizing Fe–Mn–Cr metasomatism process incorporating water, with the calcium being utilized in the formation of the augite, kirschsteinite, and diopside-hedenbergite. This druse formation scenario is consistent with a
pneumatolytic formation process. Some of the vugs are now filled with glass.
Ubiquitous primary glasses present in D’Orbigny have unfractionated chondritic relative abundances of refractory lithophiles, indicating a possible origin through bulk rock melting, but excluding an origin as residues of a partial melt. Solar-like trapped
noble gasesElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More present in these glasses are thought to have originated from primordial reservoirs of
solar windSupersonic flow of high-speed charged particles continuously blowing off a star (mostly e- and p+). When originating from stars other than the Sun, it is sometimes called a "stellar" wind. The solar wind may be viewed as an extension of the corona into interplanetary space. The solar wind emanates radially gases which accumulated very early in
Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. history. These noble gases were subsequently implanted within the glasses by way of an ascending deep magma (Busemann
et al., 2006). The presence of vesicles in Sahara 99555 and D’Orbigny angrites attests to this rapid ascent and cooling of volatile-enriched magma. Schiller
et al. (2010) argue that angrites experienced such
volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). depletion associated with
accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More within a short time after
CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More formation; timing of angrite volatile depletion was ~1 m.y. before volatile depletion occurred on the HED
parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More. Two further episodes of volatile depletion on the angrite parent body are considered likely impact-related events. Formation of the glasses was contemporaneous with formation of the bulk of D’Orbigny.
An alternative formation mechanism for the glass phase has been proposed by Varela
et al. (2003). Based upon the finding that some elemental abundances, such as FeO and MnO, as well as some elemental ratios, such as CaO/TiO and FeO/MnO, are similar to those in CI
chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, and because the glass shares many characteristics with glass inclusions in olivine grains from carbonaceous chondrites (
e.g., the presence of
volatile elementsChemical elements that condense (or volatilize) at relatively low temperatures. The opposite of volatile is refractory. Volatile elements can be divided into moderately volatile (Tc = 1230–640 K) and highly volatile (Tc < 640 K). The moderately volatile lithophile elements are: Mn, P, Na, B ,Rb, K, F, Zn. The moderately Click on Term to Read More such as C and N, thought to have been incorporated as refractory material, which were subsequently volatilized through
oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More reactions and the depletion of volatile lithophile elements), they proposed that the glass crystals grew from a vapor phase (conceivably in the
solar nebulaThe primitive gas and dust cloud around the Sun from which planetary materials formed.) upon moist surfaces within
interstitialTerm applied to ions or atoms occupying sites between lattice points. Click on Term to Read More spaces during olivine formation. Later, an oxidizing metasomatic alteration event that was intrinsically chondritic affected the glass and bulk rock.
NitrogenPrincipal constituent of the Earth’s atmosphere (78.08 vol. % at ground level). Nitrogen is the fifth most abundant element in the universe by atom abundance. Nitrogen comprises only 3.5 vol. % of the atmosphere of Venus and 2.7 vol. % of Mars’s atmosphere. Nitrogen has two isotopes: 14N (99.632 %) and 15N Click on Term to Read More in D’Orbigny is scarce and exhibits an enrichment in δ
15N (Abernethy
et al., 2013). Futhermore, D’Orbigny has a low abundance of C that is also enriched in the heavier isotopes (δ
18O) compared to other angrites, demonstrating a preferential loss of lighter isotopes during degassing. This enrichment in δ
18O is unique from all other angrites, but similar to CI/CM chondrites. Although they could not determine a specific correlation between the C and N based either on their abundances or isotopic compositions, it was demonstrated that much of the C and N was likely incorporated as atoms within the silicate
latticeRegularly spaced array of points that represents the structure of a crystal. Crystals are composed of groups of atoms repeated at regular interval in three dimensions with the same orientation. The smallest division of the lattice which can still be used to represent the entire structure is called the unit Click on Term to Read More, probably attained through metasomatic processes involving sulfur-rich fluids. It was further hypothesized that the atomic C originated from
graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More, itself being an earlier product of a
carbonateMineral or compound containing carbon and oxygen (i.e. calcium carbonate, CaCO3, calcite). Click on Term to Read More reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More process, or that it was a result of dissociation of CO and CO
2. In a similar manner, it was shown that atomic N was likely dissociated at high temperatures and then became bound within the silicate lattice. There remains a speculation at this point that some of the C and/or N was originally an
organicPertaining to C-containing compounds. Organic compounds can be formed by both biological and non-biological (abiotic) processes. Click on Term to Read More component of a carbonaceous phase similar to that found in CM-type carbonaceous chondrites.
Prior studies have shown that the close textural and compositional trends present in the angrites D’Orbigny, Sahara 99555, Asuka 881371, LEW 87051, NWA 1670, and possibly NWA 1296 provide evidence for their crystallization from a common magma source (see also the CRE age data below). It was suggested that this D’Orbigny group of angrites underwent rapid cooling and crystallization at depths of less than 0.5 m. However, since D’Orbigny contains no solar implanted gases, it could not have been exposed to the surface environment of the parent body. Angra dos Reis, LEW 86010, and NWA 2999 show evidence of a slower cooling history than the angrite grouping above, and they are probably not co-magmatic with them. Furthermore, precise U–Pb ages obtained for these three slowly cooled angrites indicate that they crystallized at least 0.9 m.y. apart, inferring an independent source magma for some or all of them (Amelin, 2007).
The results of CRE age studies based on cosmogenic
nuclideA nuclear species characterized by Z protons and N neutrons. Click on Term to Read More data infer a CRE age for D’Orbigny of 12.3 (±0.9) m.y. (Eugster
et al., 2002); this age is significantly different from all other angrites studied. Multiple episodes of impact, disruption, and dissemination of the
crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More can be inferred by the wide range of CRE ages determined for the angrites—<0.2–56 m.y. for thirteen angrites measured to date, possibly representing as many ejection events (Nakashima
et al., 2008; Wieler
et al., 2016; Nakashima
et al., 2018). This range is consistent with a single large parent body enduring multiple impacts over a very long period of time, which would suggest that the parent object resides in a stable
orbitThe elliptical path of one body around another, typically the path of a small body around a much larger body. However, depending on the mass distribution of the objects, they may rotate around an empty spot in space • The Moon orbits around the Earth. • The Earth orbits around Click on Term to Read More (planetary or
asteroid beltBelt located between 2.12 and 3.3 AU from the Sun and located between the orbits of Mars and Jupiter containing the vast majority of asteroids. The asteroid belt is also termed the main asteroid belt or main belt to distinguish it from other asteroid populations in the Solar System such Click on Term to Read More) permitting continuous sampling over at least the past 56 m.y. Alternatively, Nakashima
et al. (2018) consider it plausible that there is currently at least two angrite (daughter) objects occupying distinct orbits: one representing the fine-grained (quenched) angrites with the shorter CRE age range of <0.2–22 m.y., and another representing the coarse-grained (
plutonicGeology: Igneous intrusive body that forms when magma is injected into host rocks and solidifies. Plutons occur in the crust of asteroids undergoing differentiation or planets. Named after Pluto, the Roman god of the underworld. Plutonic rocks are the rocks found within a pluton. Astronomy: Category of planet including all Click on Term to Read More) angrites with the longer CRE age range of 18–56 m.y. (see diagram below). Cosmic-ray Exposure Ages of Angrites
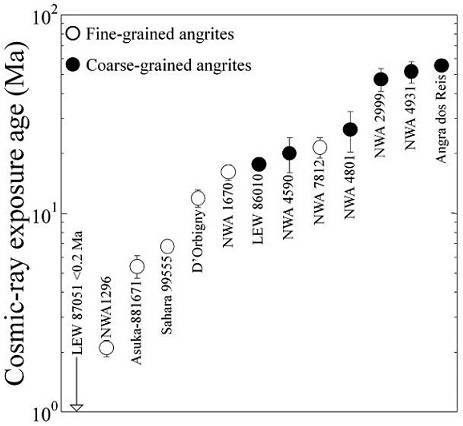
Diagram credit: Nakashima
et al.,
MAPS, vol. 53, #5, p. 965 (2018)
‘Noble gases in angrites Northwest Africa 1296, 2999/4931, 4590, and 4801: Evolution history inferred from noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More signatures’
(http://dx.doi.org/10.1111/maps.13039) Trace
elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More data argues for a more complex history for D’Orbigny and most angrites, including a non-igneous formation from a refractory
condensateIn the solar nebula, product of a chemical condensation reaction where a mineral phase precipitates (condenses) directly from a cooling vapor. Click on Term to Read More of a chondritic nature. Late phases of D’Orbigny are enriched in moderately volatile elements compared to early phases, and the two phases were formed under very different
redoxOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions—the early phases grew under highly
reducingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions while the late phases grew under highly oxidizing conditions (Varela
et al., 2005). The occurrence within anorthite (with or without olivine) of metal+sulfide arrays having a high Ni content (up to 50%) provides further evidence for a reducing sulfurous environment in the early formation history of D’Orbigny (Varela
et al., 2015). Moreover, Hwang
et al. (2015) observed FeS–oxide associations hosted by anorthite which indicate an increasing degree of oxidation over time. It is also reported that the highly incompatible elements in all olivine phases are far out of
equilibriumTerm used to describe physical or chemical stasis. Physical equilibrium may be divided into two types: static and dynamic. Static equilibrium occurs when the components of forces and torques acting in one direction are balanced by components of forces and torques acting in the opposite direction. A system in static Click on Term to Read More (highly enriched) with the parental melt that formed the bulk rock. Curiously,
plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More, which formed together with the olivine, contains very different abundances of incompatible elements, suggesting that the olivine and plagioclase formed from melts of dissimilar compositions. It is also considered that the solid spheres, which are now enriched in Ca and unfractionated trace elements, may have previoulsy been composed of CaS before undergoing decomposition under increasingly high oxidizing conditions. By this process the previously bound trace elements were converted into a vapor phase and became available for late phase metasomatism and augite formation.
Angrites are extremely ancient meteorites, with some such as D’Orbigny having accretion ages as early as ~0.5 m.y. after the first nebular condensates (
CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More) were formed (Sugiura and Fijiya, 2012). Other angrites such as LEW 86010 and Angra dos Reis attest to the fact that basaltic extrusion on the angrite parent body continued for ~7 m.y. longer. The early thermal history of the angrite parent body is most consistent with a relatively large sized planetesimal of at least 100 km in diameter (Sahijpal
et al., 2007). One scenario for the formation of angrites involves an igneous history.
- From formation models developed by Sahijpal et al. (2007), and from Rb–Sr and Hf–W systematics ascertained by Hans et al. (2009), it can be inferred that the angrite parent body experienced a relatively early onset of accretion, which was associated with volatile loss, within ~2–3 m.y. of Solar SystemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with history, a process which then proceeded rapidly to completion over a timeframe of <10 t.y. Crystallization of the angrites proceeded as a two-stage process, beginning with partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More from a CV-like chondritic source composed of olivine, orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More, and clinopyroxene at low pressures and elevated oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More levels. The abundance of H2O in the parental magma as calculated by Suzuki et al. (2012) was ~0.003–0.012 wt%. They inferred an APB mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More H2O content of ~0.001–0.003 wt% (= 10–30 ppm) based on ~30–40% partial melting and fractionationConcentration or separation of one mineral, element, or isotope from an initially homogeneous system. Fractionation can occur as a mass-dependent or mass-independent process. Click on Term to Read More. Heat generated by the decay of short-lived radiogenic isotopes produced metal–silicate melting, differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More, and basaltic melt extrusion within ~100 t.y. of the onset of accretion. This basaltBasalt is the most common extrusive igneous rock on the terrestrial planets. For example, more than 90% of all volcanic rock on Earth is basalt. The term basalt is applied to most low viscosity dark silicate lavas, regardless of composition. Basalt is a mafic, extrusive and fine grained igneous rock Click on Term to Read More was slowly cooled to ~650°C, while some of the melt experienced rapid quenching (7–13°C/hr) during eruption onto the surface and/or through a severe impact event.
Based on a study of highly volatile elements (e.g., H, C, F) in the D’Orbigny and Sahara 99555 angrites, Sarafian et al. (2017) determined that H2O and C are enriched by a factor of one million based on the observed abundances of moderately volatile elements, the latter exhibiting relatively high depletions either inherited from the nebulaAn immense interstellar, diffuse cloud of gas and dust from which a central star and surrounding planets and planetesimals condense and accrete. The properties of nebulae vary enormously and depend on their composition as well as the environment in which they are situated. Emission nebula are powered by young, massive Click on Term to Read More or associated with planetesimal accretion. They posit that 0.1–1 wt% of volatile-rich carbonaceous chondrite-type material was added to the APB sometime between the time of coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More formation ~4.5650 ago (consistent with chondritic HSE ratios) and the time of crystallization of the earliest known angrites ~4.5636 b.y. ago. From this amount of carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More material they infer an APB mantle H2O content of ~230 ppm (= 0.023 wt%).
- Severe outgassing of volatiles occurred during the impact event(s), possibly hastened by the reduced strength of the gravitational field of the fragmented planetesimal.
- A magnetic field with a strength ~20% that of present-day Earth was imparted to the angrite PB during its earliest phase of crystallization (as observed from D’Orbigny); this magnetic field may possibly be attributable to an orbital residence very near to the early T-Tauri phase solar field, or to an internal core-dynamo mechanism (Weiss et al., 2008).
- Based on studies of how kirschsteinite-lamellae profiles relate to cooling rates, as well as results of crystallization experiments, the burial depth of the angrites as they were rapidly crystallized in a thin lavaHot molten or semifluid rock derived from a volcano or surface fissure from a differentiated and magmatically active parent body. Click on Term to Read More flow is inferred to have been within 1 m of the surface.
Another possible petrogenetic history involves a non-igneous formation:
- Kurat et al. (2004) and Varela et al. (2005) have conducted extensive studies of D’Orbigny and other angrites in which they utilized mutiple sources of data (e.g., structural, textural, chemical, and redox evidence). They concluded that the angrites are most consistent with a non-igneous origin from refractory solar nebula condensates having chondritic abundances—basically an asteroid-sized version of a CAI—which record unusual circumstances (e.g., changing redox conditions) in the early history of the solar system.
A number of whole-rock and
mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More isochrons have been calculated for the angrites. A U–Pb age of 4.5553 (±0.0017) b.y. was previously reported for D’Orbigny (Jotter
et al., 2002), an age that is slightly younger than that determined for other angrites using this isotopic system (4.5578 [±0.0005] b.y. for LEW 86010 and AdoR). Other studies of
matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More and druse pyroxenes from D’Orbigny have yielded a range of U–Pb ages between 4.549 (±0.002) b.y. and 4.563 (±0.001) b.y (Jagoutz
et al., 2003), with a mean age of 4.5639 (±0.0006) b.y. (Zartman
et al., 2006). A more precisely determined measurememnt of the Pb–Pb isotopic ages yielded an even older age for D’Orbigny of 4.56442 (±0.00012) b.y. (Amelin, 2007). These ages are consistent with the Pb–Pb age determined for the A-881371 angrite (4.5624 [±0.0016] b.y.). Work to more precisely resolve the initial
238U/
235U ratio, previously accepted to be 137.88, has been ongoing. A value of 137.822 (±0.028) was calculated by Brennecka
et al. (2010), and even more precise values of 137.777 (±0.013) and 137.794 were calculated by Iizuka
et al. (2014) and Goldmann
et al. (2015), respectively. When the value of Iizuka
et al. (2014) is substituted for the previous value, the U-corrected Pb–Pb ages of D’Orbigny and Sahara 99555 are found to be identical within uncertainties at 4.56337 (±0.00025) b.y. and 4.56353 (±0.00014) b.y., respectively (Tang and Dauphas, 2014 and references therein). However, high precision U-isotopic analyses conducted by Tissot
et al. (2016) for six angrites (NWA 4590, NWA 4801, NWA 6291 [= NWA 2999], Angra dos Reis, D’Orbigny, and Sahara 99555) revealed that some heterogeneity exists in the δ
238U values among them, which demonstrates that further correction will be needed to obtain precise Pb–Pb ages for these angrites (this dating to follow). Other isotopic chronometers, such as Ar–Ar, Sm–Nd, and Lu–Hf, provide anomalous ages inconsistent with the U-corrected Pb–Pb age, which reflects late secondary processes such as impacts on the angrite parent body (Bouvier
et al., 2015).
In addition, Glavin
et al. (2004) calculated an absolute Mn–Cr isotopic age for D’Orbigny of 4.5629 (±0.0006) b.y., which is concordant with the Al–Mg age calculated by Spivak–Birndorf
et al (2005, 2009) for both D’Orbigny and Sahara 99555, as well as the Mn–Cr ages and Hf–W ages determined for both angrites (Nyquist
et al., 2003; Spivak–Birndorf
et al, 2009). Utilizing precise Al–Mg and Hf–W chronometry, Kruijer
et al. (2014) calculated a similar formation age for D’Orbigny and Sah 99555 of ~4.8 m.y. after CAIs. Each of these extinct radionuclides provide formation ages that are slightly younger than the measured Pb–Pb ages.
The ~4.564 b.y. Pb–Pb age for D’Orbigny is ~7 m.y. older than some other angrites such as AdoR, LEW 86010 and NWA 2999 (~4.5578, ~4.558, and ~4.557.9 b.y., respectively), and attests to very early accretion, igneous activity, differentiation, partial melting, and production of basaltic magma on the planetesimal. Attainment of isotopic equilibrium and crystallization will have occurred very soon thereafter (Shukolyukov and Lugmair, 2007). The decay products of extinct radionuclides such as
53Mn,
146Sm,
244Pu, and
182Hf suggest that the entire sequence from nebular condensation through parent body accretion, partial melting, siderophile–
lithophile elementElement that tends to be concentrated in the silicate phase, e.g., B, O, halogens, alkali earths, alkali metals, Al, Si, Sc, Ti, V, Cr, Mn, Y, Zr, Nb, REE, Hf, Ta, W, Th, and U. fractionation, multiple metasomatic alteration events, and final cooling to temperatures low enough to retain
fissionBreaking apart of a body into smaller fragments. In nuclear physics, fission refers to splitting of a heavy atomic nucleus into two or more lighter nuclei with an associated release of energy. The mass of the nucleus before fission is greater than the combined masses of the resulting fragments; the Click on Term to Read More tracks and noble gases was on the order of only a few m.y.
Trace and major element compositions and textures of D’Orbigny and Sah 99555 are almost identical (Nyquist
et al., 2003; Floss
et al., 2003), suggesting a possible genetic relationship (
i.e., same parent body). In addition, Asuka 881371 and LEW 87051 have trace element trends similar to D’Orbigny and Sah 99555, suggesting that they may all share a common origin, or at least experienced similar petrologic histories. Trace element trends for LEW 86010 and AdoR are significantly different from each other and from all other angrites, and they represent distinct lithological sources.
Spectral data from studies of these new angrites, especially D’Orbigny, have yielded two possible spectral analogs among main-belt asteroids: the A-type 289 Nenetta and the Sr-type 3819 Robinson. Both asteroids exhibit the strong spectral reddening characteristic of the Al,Ti–diopside-hedenbergite component of angrites. However, important differences exist—the spectra of 289 Nenetta and 3819 Robinson contain distinct olivine bands which are absent in that of D’Orbigny, and the spectra of 3819 Robinson matches that of D’Orbigny in the visible but not in the near-infrared. It was inferred by Nyquist and Bogard (2003) that since D’Orbigny was spectroscopically similar to these two asteroids, both located between ~2.8 and 2.9
AUThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More, it was also probable that the angrite parent body formed in this same region. They argued that asteroids at this
heliocentricCentered around a sun. Our own Solar System is centered around the Sun so that all planets such as Earth orbit around the Sun. Note that 25% of Americans incorrectly believe the Sun revolves around the Earth. Click on Term to Read More distance accreted too slowly to permit the accumulation of enough radiogenic
26Al to cause global melting and differentiation before attaining a diameter greater than ~200 km;
i.e., a body larger than ~200 km in diameter would not have produced enough radiogenic heat to melt and differentiate an object of this size. By this line of reasoning, it could be concluded that the differentiated angrite PB was either not as large as 200 km in diameter, or that it formed at a smaller heliocentric distance than ~2.8 AU.
Without regard to heliocentric distance, Sanders and Scott (2007) argued that any body that accreted to a diameter >60 km (
i.e., large enough to minimize heat loss from the surface through
conductionTransfer of heat as a result of collisions between molecules; when one end of an object is heated or excited, the molecules vibrate faster and their energy is transferred sequentially to their neighbors. Click on Term to Read More) within ~2 m.y. of CAI formation (the oldest objects in the Solar System, dating to 4.567 b.y. ago) as the angrites did, must contain enough
26Al to produce global melting and differentiation. In contrast, Senshu and Matsui (2007) reasoned that accretion to a diameter of only ~14 km occurring within 2 m.y. of CAI formation was all that was required for global differentiation to occur, while accretion to a diameter of 40–160 km within 1.5 m.y. after CAI formation was cited by Hevey and Sanders (2006) and Sanders and Taylor (2005) as the minimums. Sanders and Scott (2011) later revised that to suggest radiogenic melting proceeded in bodies >20 km in diameter when accreted within 1.5 m.y. after CAI formation, while bodies accreting later than 1.5 m.y. after CAIs were heated but not melted. Furthermore, they found that bodies which accreted later than 2.2 m.y. would not have melted at all. Nevertheless, it can still be represented that at large heliocentric distances (>~2.8 AU), accretion would proceed too slowly for sufficient
26Al to accumulate and initiate global melting prior to a body growing too large (~200 km diameter) for melting to be possible (Nyquist and Bogard, 2003).
John T. Wasson (2016) presented evidence that the slow heating generated entirely by the decay of
26Al is insufficient to melt asteroids, and that an additional heat source would have been required;
e.g., the rapid heating incurred from major impact events. He determined that the canonical
26Al/
27Al ratio of 0.000052 is much too low to cause any significant melting, and that a minimum ratio of 0.00001 would be required to produce a 20% melt fraction on a well-insulated body having a significant concentration of
26Al. For example, the initial ratio of 0.0000004–0.0000005 calculated for the angrites Sah 99555 and D’Orbigny based on their
26Al–
26Mg isochrons is too low to have generated any significant melting without an additional heat source. Therefore, impacts were a major source of heating in early solar system history. It has also been suggested by some that relatively small
planetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More might have been just the required size to allow heating by induction in the
plasmaFourth state of matter: a gas in which many or most of the atoms are ionized. In the plasma state the atoms have split into positive ions and negative electrons, which can flow freely, so the gas becomes electrically conducting and a current can flow. Click on Term to Read More environment of the T Tauri
SunOur parent star. The structure of Sun's interior is the result of the hydrostatic equilibrium between gravity and the pressure of the gas. The interior consists of three shells: the core, radiative region, and convective region. Image source: http://eclipse99.nasa.gov/pages/SunActiv.html. The core is the hot, dense central region in which the.
The spectrum of asteroid 3628 Božněmcová has also been studied and compared to those of the angrite meteorites (Cloutis
et al., 2006). Božněmcová is thought to have experienced partial melting and
fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More under oxidizing conditions, and is considered to have a surface composition akin to an angritic crust (
i.e., a composition of ~55–75 wt% clinopyroxene, ~20–33 wt% plagioclase
feldsparAn alumino-silicate mineral containing a solid solution of calcium, sodium and potassium. Over half the Earth’s crust is composed of feldspars and due to their abundance, feldspars are used in the classification of igneous rocks. A more complete explanation can be found on the feldspar group page. Click on Term to Read More, and 0–20 wt% olivine plus kirschteinite). It is a spectral type A asteroid containing an Fe
+3-free clinopyroxene phase known only from angrites. However, despite its similarities in reflectance spectra, and thus mineralogy, to that of angrite meteorites, the latter typically contain more olivine than is observed on Božněmcová. On the other hand, studies of the orbits of the LL6 ordinary chondrites Bensour and Kilabo (Alexeev
et al., 2009) suggest that these meteorites cross the orbit of Božněmcová, which is located in the inner asteroid belt (~2.2 AU). This location is associated with two efficient resonances responsible for transferring material into Earth-crossing trajectories.
Portions of the angrite asteroid must be in a stable orbit (planetary or asteroid belt) from which
spallationThe formation of new nuclides by interactions of high-energy cosmic ray protons with target nuclei that commonly produce several smaller product nuclides. has continued to occur over the past ~56 m.y. as indicated by the wide variation in angrite CRE ages. Notably, Rivkin
et al. (2007) have determined that the largest known co-orbiting Trojan asteroid of Mars, the 1.3 km-diameter 5261 Eureka located at a trailing Lagrangian point, is a potentially good spectral analog to the angrites (as measured by Burbine
et al., 2006) (see diagrams below). They suggest that 5261 Eureka could represent a captured fragment of the disrupted angrite parent body now in a stable orbit around Mars.
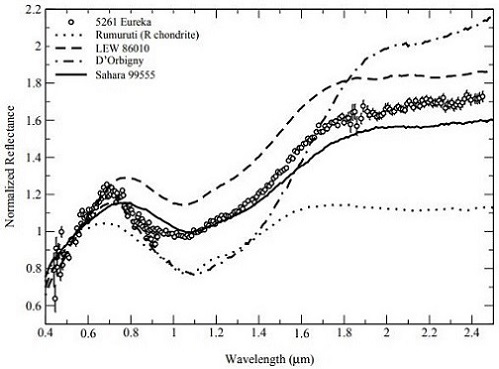
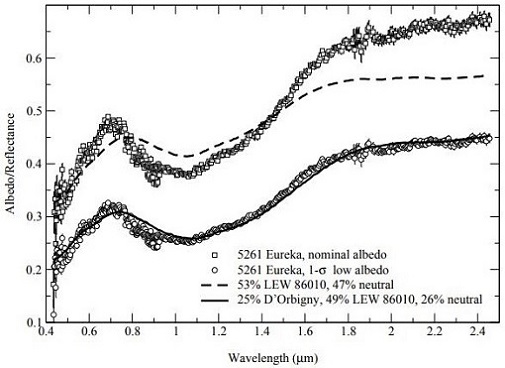
Diagrams credit: Rivkin
et al.,
Icarus, vol. 192, #2, (2007)
‘Composition of the L5 Mars Trojans: Neighbors, not siblings’
(https://doi.org/10.1016/j.icarus.2007.06.026; open access link) The number of unique angrites represented in our collections today is limited, and they have been grouped by some as basaltic/quenched, sub-volcanic/metamorphic, or plutonic/metamorphic, along with a single dunitic sample in NWA 8535 (
photo courtesy of Habib Naji). Notably, another
findMeteorite not seen to fall, but recovered at some later date. For example, many finds from Antarctica fell 10,000 to 700,000 years ago. Click on Term to Read More from Antarctica, Y-1154, is an anomalous meteorite containing Al,Ti–diopside-hedenbergite that is compositionally similar to angrites, but it has a unique fine-grained, dendritic texture. An excellent petrographic
thin sectionThin slice or rock, usually 30 µm thick. Thin sections are used to study rocks with a petrographic microscope. micrograph of D’Orbigny can be seen on
John Kashuba’s webpage. The specimen of D’Orbigny pictured above is a 1.6 g partial slice.










