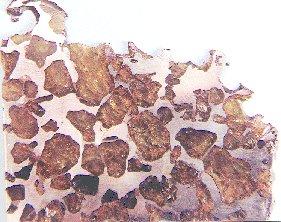
Imilac
PallasiteOne of two main classes of stony-iron meteorite, the other being mesosiderites. Pallasites are igneous in nature and characterized by crystals of olivine, sometimes peridot (green gem quality clear olivine crystals), embedded in a matrix of Fe-Ni metal. The type specimen, weighing 680 kg, was found in the mountains near Click on Term to Read More, MG (main-group)
high-Δ17O subgroup
Found 1820
24° 12.2′ S., 68° 48.4′ W. In 1828, some small Imilac specimens were obtained on behalf of the British and Royal Scottish Museums in Buenos Aires from an Indian, José MariaBroad low plains surrounded by basin-forming mountains, originally thought to be a sea (pl. maria). This term is applied to the basalt-filled impact basins common on the face of the Moon visible from Earth. Click on Term to Read More Chaile. He had found the first specimens in the Atacama Desert southwest of Imilac, Chile in about 1820, and had traveled through the Atacama Desert and the Andes Mountains to sell the specimens in the capital city. In January of 1854, a professor in Santiago named Philippi was shown the strewnfield by Chaile, where he recovered numerous small specimens weighing ~4.5 kg. He also identified a hole 6 m deep thought to have been excavated by Indians searching for the supposed metallic vein. The largest mass of 198.1 kg was purchased from an Indian for the British Museum in 1877. In the intervening years thousands of smaller fragments were recovered such as the intricately patterned specimen pictured below weighing only 4.0 g.

These pallasite masses have been perfectly preserved in the extremely dry environment of the Andes Mountains. The meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More is composed of equal parts olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More and FeNi-metal. The yellow- to orange-colored, angular, highly fractured olivine crystals have an average size of 10 mm, but some are twice as large. The metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More in the smaller specimens shows evidence of violent shearing and deformation, with frictional heating reaching recrystallization temperatures. No Thomson (Widmanstätten) structure is present on etched sections. Recent work by Killgore and McHone (1997), using modern navigation aids, has revealed the existence of a pattern of rays of fragments emanating from the east side of an 8-m impact pit. Two smaller depressions located in line with the large pit show evidence which suggests they also were formed by an impact from an object approaching from the west-southwest, defining a strewnfield of 400 m × 200 m. Erosional forces transported many of the smaller masses downhill to the southeast. Three tenable scenarios for the formation of the main-group pallasites are presented here, while other plausible hypotheses are outlined below. The first scenario utilizes a passive mechanism to explain the silicate–metal mixing, the second envisions an impact-induced injection of molten metal into olivine at a near-surface location, and the third proposes that a glancing impact disrupted a smaller body, which was followed by its reassembly into a pallasite parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More. Scenario 1 (e.g., Boesenberg et al., 2012; Donohue et al., 2018)
- Olivine crystallized from the silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the liquid at the lowest layer of the mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More, the core–mantle interface.
- Cooling and contraction of the metallic coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More produced a 2% volume reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More leaving a voidHuge region of space that is unusually empty of galaxies. Voids are not entirely empty, but are underdense and contain far fewer bright galaxies than average. at the core–mantle boundary.
- The overlying crystalline olivine then collapsed into the viscous metal where heating and mixing occurred to produce the pallasitic structure.
- Rounding of olivine crystals, once considered to be due to long-term annealing (Saiki et al., 2003), is now thought to have occurred primarily from resorption at high temperatures (above ~1250°C) in the presence of silicate melt (Boesenberg et al., 2012); a Thomson (Widmanstätten) structure was developed in the FeNi-metal component.
- Boesenberg et al. (2012) propose a model in which the formation of a pallasite layer is the result of progressive fractional melting of a chondritic body. Over time, metal–silicate separation occurs producing an insulating crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More and regolithMixture of unconsolidated rocky fragments, soil, dust and other fine granular particles blanketing the surface of a body lacking an atmosphere. Regolith is the product of "gardening" by repeated meteorite impacts, and thermal processes (such as repeated heating and cooling cycles). Click on Term to Read More along with a sulfide-rich metallic core, and in the molten outer core, buoyant olivine crystals coalesce and form a dunite layer. Heat from the molten core causes convectionTransfer of heat energy by moving material. Temperatures increases with depth in planetary objects. Deep hot less-dense material physically rises and cools, releasing heat and becoming denser. The now cooler denser material sinks back into deeper regions, where it will be reheated and rise again. Convection is an important mechanism Click on Term to Read More in the overlying mush of olivine+silicate melt+molten metal, wherein olivine near the dunite layer is entrained in the molten metal which promotes the downward crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More of metal forming a pallasitic assemblage. A less significant role was inferred for impacts, by which shock waves produce fragmentation of some olivines; some fragmental olivines are subsequently rounded through partial resorption within the silicate melt.
In a subsequent experiments, Donohue et al. (2018) expanded upon this fractional melting model (see diagram below). They contend that as temperatures decrease from peak values of ~1600–1700°C to ~1000°C, at rates of ~100–300°C/m.y., minor phases crystallize through redoxOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More reactions and grain boundary diffusionMovement of particles from higher chemical potential to lower chemical potential (chemical potential can in most cases of diffusion be represented by a change in concentration). Diffusion, the spontaneous spreading of matter (particles), heat, or momentum, is one type of transport phenomena. Because diffusion is thermally activated, coefficients for diffusion Click on Term to Read More. Partial equilibration occurred over a timescale of a few million years, altering the elemental distribution among the olivine, metal, and minor phases. Phosphorus from the molten metal was taken up into the residual silicate melt and ultimately formed phosphates (in a cooling sequence of merrillite, stanfieldite, farringtonite, Fe-rich phosphate, and silico-phosphate), with phosphoran olivine remaining as melt is exhausted. OrthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More, chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More, and schreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More were also formed as late-stage phases. Cooling rates gradually decreased to relatively slow rates of ~1°C/m.y. as determined through metallographic cooling models.
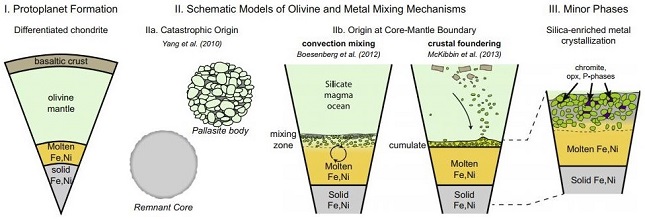
click on image for a magnified view Diagram credit: Donohue et al., GCA, vol. 222, p. 315 (2018)
‘Experimentally determined subsolidus metal-olivine elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More partitioningThe tendency of elements to prefer one mineral phase relative to another or to preferentially enter the solid or remain in the liquid during crystallization. Click on Term to Read More with applications to pallasites’
https://doi.org/10.1016/j.gca.2017.10.030
Scenario 2 (Hsu, 2003)
- Olivine crystallized as a fractionationConcentration or separation of one mineral, element, or isotope from an initially homogeneous system. Fractionation can occur as a mass-dependent or mass-independent process. Click on Term to Read More cumulateIgneous rock composed of crystals that have grown and accumulated (often by gravitational settling) in a cooling magma chamber. Click on Term to Read More from the silicate liquid in a magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More chamber (as suggested by the lack of a trapped melt component, and consistent with elemental trends), or as a partial melt residue; ~50–70% melting is indicated
- A high-energy impact(s) resulted in the high-pressure injection of low-viscosity metal into the crystalline olivine layer.
- The pallasite material experienced very rapid cooling at high temperatures and slow cooling at low temperatures, consistent with the preservation of separate olivine and FeNi-metal and of zoning profiles (e.g., Ni) in the olivine.
- Evidence of live 53Mn, as well as other chronometric data, indicates that pallasites were formed within the first 10 m.y. of solar sytem history.
- A later event(s) produced an extensive regolith, which buried the pallasite material and initiated a period of slow cooling.
- Rounding of olivine crystals, once considered to be due to long-term annealing (Saiki et al., 2003), is now thought to have occurred primarily from resorption at high temperatures (above ~1250°C) in the presence of silicate melt (Boesenberg et al., 2012); a Thomson (Widmanstätten) structure was developed in the FeNi-metal component.
- The olivine-metal mixing event could have resulted from the impact of a differentiated body having a fractionated liquid iron core onto another differentiated protoplanetary object very early in Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. history—as early as ~4.557 b.y. ago and not later than ~4.3 b.y. ago. The injection of molten metal from the impactor created impact-melt, dike-like intrusions in the cold olivine mantle of the host body, forming a pallasitic mixture that was first rapidly frozen and then slowly cooled over a period of at least several tens of millions of years. Isotopic data suggest that this main-group pallasite parent body formed in the terrestrial planet-forming region. Thereafter, one or more severe impacts sent pallasitic fragments into parking orbits within the asteroid beltBelt located between 2.12 and 3.3 AU from the Sun and located between the orbits of Mars and Jupiter containing the vast majority of asteroids. The asteroid belt is also termed the main asteroid belt or main belt to distinguish it from other asteroid populations in the Solar System such Click on Term to Read More. A study conducted by Tarduno et al. (2012) is most consistent with scenario 2. Paleointensity data were obtained by from sub-µm to µm-sized, stable magnetic inclusions within Imilac and Esquel olivines, continuing with analyses of Springwater (Tarduno and Cottrell, 2013). Along with cooling rate data, these inclusions indicate that pallasites formed and cooled under the influence of a strong magnetic field generated by a core dynamo on an ~320-km-diameter parent body. This remanent magnetization attests to the fact that the Imilac pallasite was not formed near the core-mantle boundary, because a rotating core dynamo would necessarily cease prior to any significant cooling of adjacent material; therefore, no remanent magnetization would exist. Their estimates of the cooling rate for pallasite material based on conductionTransfer of heat as a result of collisions between molecules; when one end of an object is heated or excited, the molecules vibrate faster and their energy is transferred sequentially to their neighbors. Click on Term to Read More (2–9K/m.y.) are consistent with a formation location within the upper ~60% of the protoplanet mantle—perhaps at depths of 10 km and 40 km for Imilac and Esquel, respectively.
- Olivine crystallized from the silicate liquid at the lowest layer of the mantle, the core–mantle interface.
- The metallic core solidified outwards until ~80 vol% crystallization was reached.
- A glancing impact disrupted the protoplanet resulting in the high-pressure injection of the residual low-viscosity metal into the crystalline olivine layer from the lower mantle.
- Diverse cooling rates reflect cooling at different depths on a common parent bodyand not at the core–mantle interface.
- A rubble-pile asteroid was formed providing a source for main group pallasites.
- Rounding of olivine crystals, once considered to be due to long-term annealing (Saiki et al., 2003), is now thought to have occurred primarily from resorption at high temperatures (above ~1250°C) in the presence of silicate melt (Boesenberg et al., 2012).; a Thomson (Widmanstätten) structure was developed in FeNi-metal regions of appropriate size.
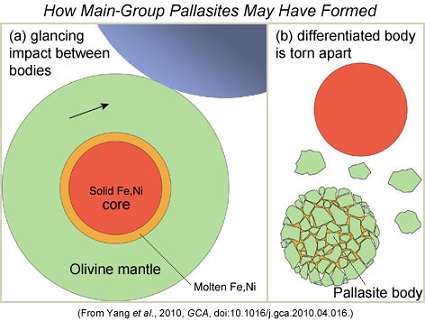
- This scenario was the basis for the PSRD article by E. Scott, J. Goldstein, and J. Yang: ‘Formation of Stony-Iron Meteorites in Early Giant Impacts‘, June 2010. The diagram shown below demonstrates the general sequence of events proposed, initiated by a glancing impact between a differentiated body and a larger object, and culminating in the reassembly of the former into a much smaller pallasite parent body.
In his study of main-group pallasites, Edward Scott (2017) expanded upon this formation model by inferring the existance of three zones at the core–mantle boundary (see diagram below). Initially, differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More of the parent body occurred forming a molten metallic core and a dunitic olivine mantle. As olivine crystallized and accumulated at the base of the mantle, some portion became immersed in the molten iron where the crystal edges underwent rounding (zone 3 in the diagram); this rounding was once considered to be due to long-term annealing (Saiki et al., 2003), but is now thought to have occurred primarily from resorption at high temperatures (above ~1250°C) in the presence of silicate melt (Boesenberg et al., 2012). Ultimately, a glancing impact disrupted the parent body resulting in fragmentation of both the early-formed, Ir-rich (0.7–5 ppmParts per million (106). Click on Term to Read More) olivine located in zone 3, and the late-formed, Ir-poor (0.01–0.3 ppm) olivine located in zones 1 and 2. During the impact event, molten iron was injected into the olivine assemblages to produce large regions of pure metal within pallasitic zones.
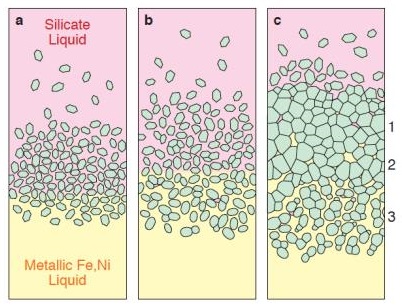
Image credit: Edward R. D. Scott, 48th LPSC, 2017, #1037
In their extensive elemental analysis of pallasites, Wasson and Choi (2003) proposed that gases associated with the metallic melt were concentrated in voids formed by core contraction and mantle collapse during cooling, and that subsequent condensation of these gases introduced enrichments of the volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). siderophiles Ge and Ga into the PMG members, as well as enrichments of Fa into the PMG-as members. They also attributed the refractory siderophile enrichments present in many pallasites (e.g., Ir) to the mixing of late-stage core metal and residual mantle metallic melts.
A study was conducted by Mittlefehldt and Herrin (2010) pertaining to the degree of magmatic fractionation of main-group pallasites, including anomalous members. They examined the Mn/Mg ratios of these pallasites and determined that there was no correlation between magmatic fractionation and metal composition. This realization was inconsistent with a core–mantle boundary origin of the olivine on a single parent asteroid. In a comparison of elemental abundances to the Mn/Mg ratios of the various pallasites, they found that the olivine was not formed through accumulation processes, but instead was formed as a residue of a high degree igneous melt. The pallasite thermal history reflects a slow cooling rate of a few degrees per million years, as evidenced by the FeNi-metal component cooling over the temperature interval of ~700°C to ~500°C, which is the interval over which the Thomson (Widmanstätten) structure is formed (Lavrentjeva, 2009). This slow cooling rate is in contrast to the much more rapid cooling rate of a few degrees per year reflected in the olivine component at high temperature conditions of ~1100°C. The olivine diffusion gradients and other thermal history details are more consistent with an impact-generated mixture of core and mantle materials than a core–mantle boundary origin. Anomalous metal and silicate compositions measured in some pallasites might reflect solid–liquid metal mixing on a single main-group pallasite parent body consistent with common O-isotopic compositions on each. Radiometric dating indicates that such an impact occurred <10 m.y. after chondruleRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More formation. A novel hypothesis addressing pallasite formation was proposed by Asphaug et al. (2006), and was adapted by Danielson et al. (2009) to account for the wide variety of metal-silicate textures and bulk compositions observed in pallasites. They assert that pallasite diversity could be attributed to their formation on a chain of objects that was produced as a result of a grazing collision between partially molten Moon- to Mars-sized planetary embryos. These may be represented by multiple disparate pallasite groups such as (i) Brenham, (ii) Imilac, (iii) Fukang, and (iv) Seymchan (Johnson et al., 2010). Uniquely similar volatile element depletions that exist between the pallasites and the HED meteorites suggest a possible association between these different planetary bodies. These facts prompt speculation that these two planetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More, while in their embryonic stages early in Solar SystemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with history, experienced a mutual grazing collision. At least as intriguing is a formation hypothesis envisioned by M. Fries (2012) in which pallasites formed in the cores of small, spherical, rapidly cooled bodies in which gravitational differentiation is at a minimum and convective forces are insignificant. Such quiescent conditions would allow silicates to remain in the core while molten metal slowly infiltrated and disaggregated the silicates into ever smaller angular fragments. A subsequent catastrophic impact disruption of the parent body sent portions of this pallasitic core into Earth-crossing orbits. The metal and O-isotopic compositions of the main-group pallasites, including the phosphoran nature of olivine in some members (Brahin, Brenham, Rawlinna 001, Springwater, and Zaisho), are consistent with features of late-stage crystallization (high-Au, ~80% core crystallization) of residual melts in the IIIAB iron core. However, recent studies appear to rule out a genetic connection to IIIAB irons and a core–mantle boundary formation scenario (Yang and Goldstein, 2006; Yang et al., 2010). New and more precise metallographic cooling rates were obtained for pallasites utilizing taeniteLess common than kamacite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Taenite, γ-(Fe,Ni), has 27-65 wt% Ni, and forms small crystals that appear as highly reflecting thin ribbons on the etched surface of a meteorite; the name derives from the Greek word for "ribbon." Click on Term to Read More Ni compositions, cloudy zone particle sizes, and tetrataenite bandwidths, the latter two parameters being positively correlated with each other and negatively correlated with the metallographic cooling rates derived from taenite. The results are not what one would expect given an origin at the core–mantle boundary. Instead, based on the size of the taenite particles (island phase) in the cloudy zone of the pallasites, as well as on the tetrataenite bandwidth, the cooling rates were demonstrated to have a wide range inconsistent with a core–mantle boundary of a solitary asteroid. Cooling rates were significantly lower for pallasites than for IIIAB irons, with rates of 2.5–18K/m.y. measured for main-group members and 13–16K/m.y. measured for the Eagle Station group, while IIIAB irons cooled at ~50–350K/m.y. This implies that the irons were actually closer to the surface than the pallasites. Paradoxically, the ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More pallasite Milton, which lacks cloudy taenite zones and did not experience shock reheating, exhibits a cooling rate >5000K/m.y. (Yang et al., 2010). In their measurement of high-Ni particles within the cloudy zone of several main-group pallasites and IIIAB irons, Yang et al. (2007) found that a correlation exists between cooling rates and bulk Ni in IIIAB irons but not in main-group pallasites. Based on the significantly larger size of the high-Ni metal particles in pallasites (82–170 nm) than in the IIIAB irons (42–58 nm), they determined that the cooling rate was ~2.5–25 times slower in the pallasites, with the wide range of cooling rates indicative of a large thermal heterogeneity within the pallasite formation zone which did not exist on the IIIAB iron parent body. Notably, the Re–Os chronometer suggests that pallasites formed 60 m.y. later than IIIAB irons, raising further doubt about a IIIAB core–mantle origin for main-group pallasites (E. Scott, 2007). Further evidence in support of separate parent bodies for main-group pallasites and IIIAB irons was provided by Huber et al. (2011). They found that pallasites have a much younger range of cosmic ray exposure (CRE) ages than the IIIAB irons. In an effort to better resolve the CRE age difference between main-group pallasites and IIIAB irons, Herzog et al. (2015) conducted highly precise cosmogenic radionuclideRadioactive isotope - Atomic nuclide that decays radioactively . Click on Term to Read More analyses of both metal and olivine components in a large number of main-group pallasites. Utilizing multiple dating systems, they demonstrated that a significant number of these pallasites define a broad cluster of ages near 100 m.y., while only a very few of the IIIAB irons measured (6 of 33; Herzog and Caffee, 2014) fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More within this range—most members of this iron group have much older CRE ages. They concluded that at least half of the main-group pallasites are associated with just a few common ejection events on their parent body, and that the IIIAB irons probably derive from a separate parent body. Previous O-isotopic analyses for main-group pallasites and the HED meteorites indicated that these two groups have values that are very similar. In a high precision comparative analysis of the oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More three-isotope composition between olivines from five main-group pallasites and representative HED samples, including eucriteMost common type of achondrite meteorite and a member of the HED group. Eucrites are basalts composed primarily of pigeonite and anorthite (An60-98). Eucrites have been placed into three subgroups based on mineralogical and chemical differences. • Non-cumulate eucrites represent the upper crust that solidified on a magma ocean after Click on Term to Read More and diogeniteDiogenites belong to the evolved achondrite HED group that also includes howardites and eucrites. They are named after the Greek philosopher Diogenes of Apollonia, of the 5th century BCE, who was the first to suggest that meteorites come from outer space (a realization forgotten for over 2,000 years). They are Click on Term to Read More material, Jabeen et al. (2013) determined that a clear distinction exists, thus demonstrating that these meteorite groups originated on separate parent bodies. In another study investigating the close O-isotopic relationship between main-group pallasites, mesosiderites, and the HED clan, Ziegler and Young (2007) discovered that non-homogenized samples of main-group pallasite olivines exhibit a bimodality in 17O values, which also distinguishes their origin from that of the mesosiderites and the HED clan. In a follow-up study, a more refined O-isotopic analysis was conducted by Greenwood et al. (2008), but their results did not support a bimodality in 17O values; however, they definitively established that the parental source of main-group pallasites was different from that of mesosiderites and the HED clan. Subsequent high-precision triple oxygen isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More analyses of a broad sampling of main-group pallasites (Brahin, Brenham, Esquel, Fukang, Giroux, Huckitta, Imilac, Seymchan, Springwater, and Sterley) and selected members of the HED group (Tatahouine, Stannern, and Juvinas) were conducted by Ali et al. (2013, 2014). Their results, together with geochemical and other data, not only demonstrate that the HEDs are not genetically related to the main-group pallasites, but also that a bimodality exists for these pallasites based on several factors: Δ17O values, MgO content in olivines, bulk olivine abundance, concentration densityMass of an object divided by its volume. Density is a characteristic property of a substance (rock vs. ice, e.g.). Some substances (like gases) are easily compressible and have different densities depending on how much pressure is exerted upon them. The Sun is composed of compressible gases and is much Click on Term to Read More of olivine grains, and paleointensity. They were able to resolve systematic variations among the main-group pallasites which indicate the existence of two distinct subgroups (see diagram below). This O-isotopic bimodality has been attributed to several possible scenarios, including the existance of multiple parent bodies, the sampling of different locations on a common parent body, and/or varibility in the degree of impactor contamination.- high-Δ17O (ave. –0.172 [±0.007] ‰), less olivine-rich (olivine/metal = 2.0); e.g., Brenham, Huckitta, Imilac, Springwater, Sterley
- low-Δ17O (ave. –0.213 [±0.011] ‰), more olivine-rich (olivine/metal = 2.9); e.g., Brahin, Esquel, Fukang, Giroux, Seymchan
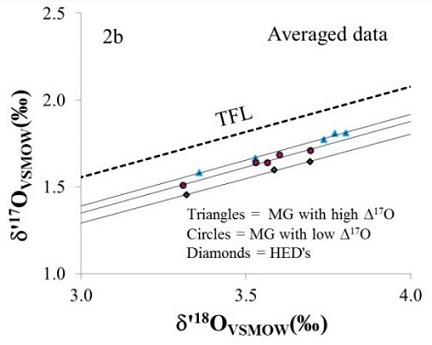
Diagram credit: Ali et al., 45th LPSC, #2390 (2014) Another high-precision oxygen isotope analysis was undertaken by Greenwood et al. (2015) in which 24 main-group pallasites (Admire, Ahumada, Brahin, Brenham, Dora, Esquel, Finmarken, Fukang, Giroux, Glorietta Mountains, Imilac, Krasnojarsk, Lipovsky, Marburg, Marjalahti, Molong, Pallasovka, Pavlodar, Quijingue, Rawlinna 001, Santa Rosalia, Somervell County, Springwater, and Theil Mountains) and a number of mesosideriteOne of two main types of stony-iron meteorite, the other being pallasites. Mesosiderites are a mixture of approximately 50% basaltic, gabbroic and orthopyroxenitic silicates and 50% Ni-Fe metal and sulfides. The name derives from the Greek "mesos" meaning "middle" or "half" and "sideros" for "iron;" hence "half-iron". The silicates are Click on Term to Read More olivine-rich clasts and related dunites (Lamont, Mount Padbury, Vaca Muerta, NWA 2968, NWA 3329) were utilized. Their results support the previous findings showing that the main-group pallasites and HED meteorites originated on separate parent bodies. However, the new Δ17O values of the 24 main-group pallasites studied do not support the previous hypothesis for bimodality, but instead indicate that a continuum exists having an average Δ17O value of –0.187 (±0.016) ‰.
- high-Δ17O-bearing (ave. –0.166 [±0.014] ‰) subgroup; e.g., Acomita, Ahumada, Brenham, Finmarken, Huckitta, Imilac, Jay Bird Springs, La’gad 002, Marjahlati, Otinapa, Pallasovka, Somervell County, South Bend, Springwater, Sterley, Thumrayt 001
- low-Δ17O-bearing (ave. –0.220 [±0.009] ‰) subgroup; e.g., Brahin, Esquel, Fukang, Giroux, Hambleton, Krasnojarsk, Mount Dyrring, Newport, Seymchan
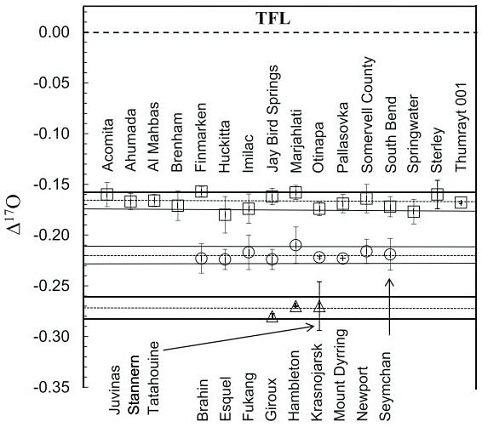
Diagram credit: Ali et al., MAPS, vol. 53, #6, p. 1228 (2018)
‘The oxygen isotope compositions of olivine in main group (MG) pallasites: New measurements by adopting an improved laser fluorination approach’
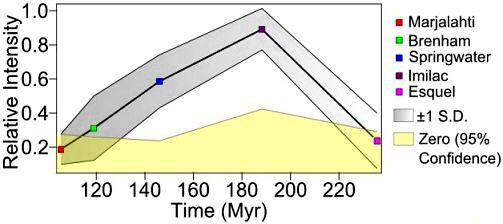
(https://doi.org/10.1111/maps.13072) Utilizing the paleointensity data of Tarduno et al. (2012) for the low-Δ17O Esquel and the high-Δ17O Imilac, Ali et al. (2014) ascertained that they each formed at different depths (40 km and 10 km, respectively) on one or more parent bodies. Paleointensity data was compiled by Nichols et al. (2018) for five pallasites representing a wide range of cooling rates (3–8°C/m.y.). These data were used to demonstrate the evolution of a late-stage core dynamo on the parent body beginning ~100 m.y. after accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More and spanning a period of ~140 m.y. (see diagram below showing relative paleointensities, where Imilac = 73.6 [±8.1] µT as determined by Tarduno et al., 2012).
Diagram credit: Nichols et al., 49th LPSC, #1976 (2018) The question pertaining to whether a genetic relationship exists among HED-clan meteorites, mesosiderites, main-group pallasites, and IIIAB irons is ongoing. It is now recognized (e.g., Sanborn et al., 2014) that a comparison of Δ17O vs. ε54Cr is one of the best diagnostic tools for determining genetic relationships between meteorite groups. Moreover, Sanborn et al. (2015) demonstrated that ε54Cr values are not affected by aqueous alteration. Utilizing both the ε54Cr and Δ17O values for representative samples of each of these meteorite groups, Wasson and Göpel (2014) found that these groups were unresolvable in terms of ε54Cr values, and that the differences in Δ17O values are reasonable given a scenario of rapid impact-heating for the HED meteorites. They argue that isotopic evidence which supports an origin of the HED meteorites on the IIIAB parent body should be considered more reliable than any association of the HED-clan meteorites with asteroid 4 VestaThird largest and fourth brightest asteroid; it was discovered in 1807 by Heinrich Olbers and named for the ancient Roman goddess of the hearth. 4 Vesta has a basaltic surface composition and an average density not much less than that of Mars. Evidently lava once flowed here indicating that the based on spectral analyses from orbitThe elliptical path of one body around another, typically the path of a small body around a much larger body. However, depending on the mass distribution of the objects, they may rotate around an empty spot in space • The Moon orbits around the Earth. • The Earth orbits around Click on Term to Read More. 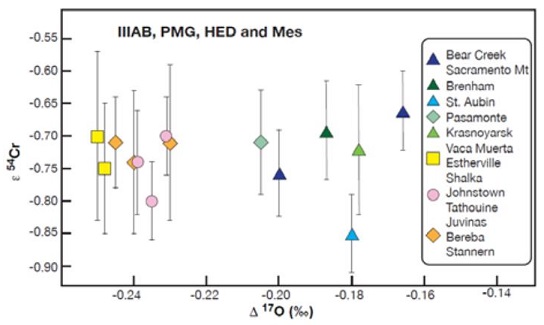
Diagram credit: Wasson and Göpel, 77th MetSoc, #5446 (2014) In an effort to better resolve potential genetic relationships that might exist among meteorite groups, a Cr-isotopic analysis was conducted by Sanborn et al. (2018) for olivine from both the main-group pallasite Brenham and the ungrouped pallasite Milton, along with the anomalous IVA irons Steinbach and São João Nepomuceno. It is demonstrated on a coupled Δ17O vs. ε54Cr diagram (shown below) that Brenham and Krasnojarsk plot significantly above the HED normal trend (black squares in inset), which supports the inference that these meteorites formed on separate parent bodies. Chromium vs. Oxygen-isotope Plot
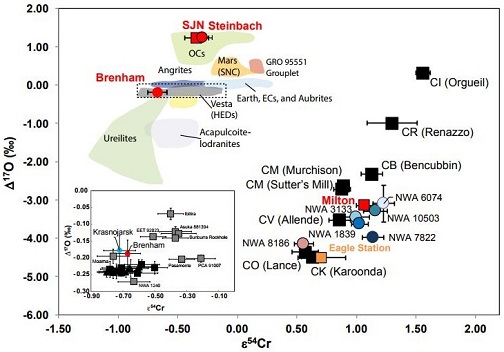
click on diagram for a magnified view

Photo courtesy of Alan Lang—R.A. Langheinrich Meteorites

Photo courtesy of Sergey Vasiliev—SV-meteorites.com