Milton
PallasiteOne of two main classes of stony-iron meteorite, the other being mesosiderites. Pallasites are igneous in nature and characterized by crystals of olivine, sometimes peridot (green gem quality clear olivine crystals), embedded in a matrix of Fe-Ni metal. The type specimen, weighing 680 kg, was found in the mountains near Click on Term to Read More, ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More
‘CX’ trend
Found October 2000
40° 17′ 15′ N., 95° 22′ 36′ W. While clearing rocks from his soybean field in Fairfax, Missouri, G. Wennihan found an unusually heavy one that had a rusty appearance. He tossed this 2,038 g rock into the back of his pickup truck to save. The large mass was eventually cut in half, and the unique appearance of the interior raised speculations that it originated in space. Eventually a friend of his who was a geology student, B. Rogers, took the strange rock to the geology department of Northwest Missouri State University where it was cleaned and examined. Although assistant geology professor Richard Felton and several faculty members examined the rock, it was Dr. Renee Rohs who recognized its resemblance to an Imilac specimen that she had seen years earlier while attending a class taught by Dr. Van Schmus (Horejsi and Cilz, 2002). Reasonably, the rock was taken to Dr. Van Schmus at the University of Kansas for his qualified opinion, and he immediately recognized that it was a pallasite. Samples of the pallasite were sent to the Institute of MeteoriticsScience involved in the study of meteorites and related materials. Meteoritics are closely connected to cosmochemistry, mineralogy and geochemistry. A scientist that specializes in meteoritics is called a meteoriticist. Click on Term to Read More at University of New Mexico and to UCLA for thorough analyses.
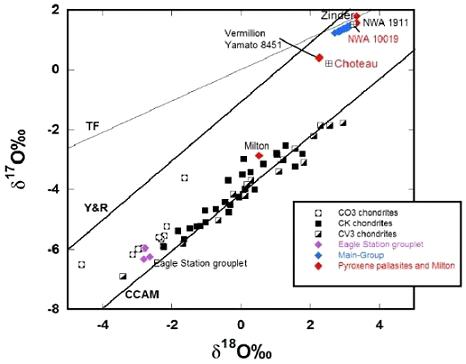
Diagram credit: Gregory et al., 47th LPSC, #2393 (2016) Some ungrouped irons have similar O-isotopic ratios to Milton but have significantly different iron chemistry, which excludes a genetic relationship. However, it was argued by Reynolds et al. (2006) that the high-Ni irons which comprise the South Byron trio (Babb’s Mill [Troost’s], South Byron, and Inland Forts [ILD] 83500) have similar metal compositions (siderophile elementLiterally, "iron-loving" element that tends to be concentrated in Fe-Ni metal rather than in silicate; these are Fe, Co, Ni, Mo, Re, Au, and PGE. These elements are relatively common in undifferentiated meteorites, and, in differentiated asteroids and planets, are found in the metal-rich cores and, consequently, extremely rare on patterns) and similar structures (kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More spindles and associated schreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More) compared to metal in Milton, and therefore these meteorites might constitute a grouplet that originated on a common parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More. Moreover, all of these irons and the metal in Milton experienced a similar oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More history during coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More formation, as evidenced by the presence of FeO-rich olivine, chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More, and phosphate, as well as the depletions in other easily oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More elements (McCoy et al., 2008). Siderophile elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More abundances for these four meteorites were shown by McCoy et al. (2017) to have very similar values. Interestingly, isotopic compositions (Mo, Ru, and W) and HSE abundances of the IVB irons and the Milton–South Byron trio grouping fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More within the range of the oxidized CV–CK chondrites (Hilton et al., 2018). Carbonaceous vs. Non-carbonaceous Irons
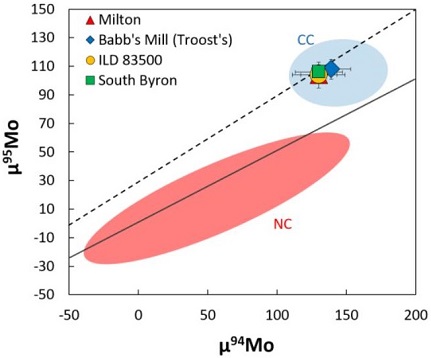
Diagram credit: Hilton et al., 49th LPSC, #1186 (2018) McCoy et al. (2017) also recognized that the presence of volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). siderophile elements in these meteorites indicates they were not derived from a high-temperature condensation process contrary to other high-Ni iron groups such as IVA, but instead oxidation (nebular or parent body) and fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More were the dominant formation processes. The Milton pallasite is a product of an early stage of fractional crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More compared to the main-group pallasites, as well as with regards to its fractional crystallization sequence among the South Byron trio irons. Based on HSE abundance patterns, Hilton et al. (2018) concluded that Babb’s Mill (Troost’s) was first in the sequence (representing the first 1% of crystallized melt) followed soon thereafter by South Byron (2%), with ILD 83500 having the highest content of incompatible P being last in the sequence (42%). If Milton is part of a core–mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More boundary then crystallization apparently proceeded inwards similar to the crystallization process some envision for the IVA and IIIAB irons following mantle removal on their respective parent bodies. In their analyses of O-isotopic composions in chromite for these four meteorites, McCoy et al. (2017) demonstrated that they plot along a similar trend line on an oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More three-isotope diagram (see below). Together with previous petrographic and geochemical data, this new O-isotopic data provides strong evidence supporting a common source parent body.
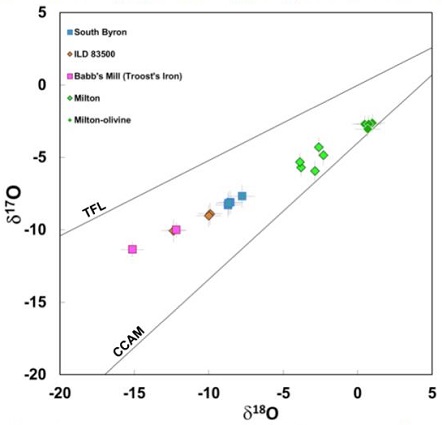
Diagram credit: McCoy et al., 48th LPSC, #2241 (2017) In addition to the irons mentioned above, several other ungrouped ataxites could be members of this high-Ni iron group, including El Qoseir, Illinois Gulch, Morradal, Nordheim, and Tucson. However, because of the significant differences that exist in their content of refractory elementsUsing research by Wood (2019), any of the elements with a relatively high condensation temperature of 1291 K < TC,50 < 1806 K in the solar nebula1. They are the first elements to condense out of a cooling gas. Refractory elements are the main building blocks of rocky planets, dwarf Click on Term to Read More compared to that in the South Byron trio, further work is needed to establish a definitive connection (Kissin, 2010). Investigators have also explored the possibility of a genetic relationship between IVB irons and other meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More groups. Based on O-isotopic analyses utilizing chromite grains from IVB irons Warburton Range and Hoba, Corrigan et al. (2017) found that IVB irons share close similarities to the Milton–South Byron trio grouping (see diagram below). The O-isotopic compositions of the IVB irons and the South Byron trio–Milton grouping also fall within the range of the oxidized CV–CK chondrites. Moreover, Corrigan and McCoy (2018) found that both IVB irons and the Milton–South Byron trio show evidence for early oxidation (e.g., both have a similar high Ni content of ~15.5–18 wt% and ~15–18 wt%, respectively), as well as evidence for late reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More (e.g., both contain reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More mineral phases such as troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More, daubréelite, and schreibersite).

Diagram credit: Corrigan et al., 48h LPSC, #2556 (2017)
Hoba: Δ17O = –3.4 [±0.2] ‰
Warburton Range: Δ17O = –3.4 [±0.4] ‰
Milton–South Byron trio: Δ17O = ~ –3.6 [±0.6] ‰ SilicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the phases in Milton are enriched in the siderophile and highly siderophile elements which typically partition into metal phases (Hillebrand et al., 2004). Because of the unusual homogeneity of its metal and silicates, Milton has served as a good tool for J. Hillebrand (2004) to determine the in situ metal/silicate partition coefficients of a pallasite. Milton is a unique representative of its parent asteroid, and demonstrates that the petrogenesis of pallasites must have occurred in a similar way on multiple parent bodies. Interestingly, the ungrouped metachondriteTerm used to describe a metamorphosed chondrite. Also referred to as a type 7 chondrite. Metachondrites are texturally evolved rocks derived from chondritic precursors and some have been classified as primitive achondrites. Click on Term to Read More NWA 10503 has been conjectured to have a possible affinity to the Milton pallasite (Irving et al., 2016). Not only does this unique meteorite share with Milton an association with carbonaceous chondrites as attested by their elevated silicate FeO/MnO ratios, but it also falls along an extension of the trend line established by Milton on an oxygen three-isotope diagram (see below).
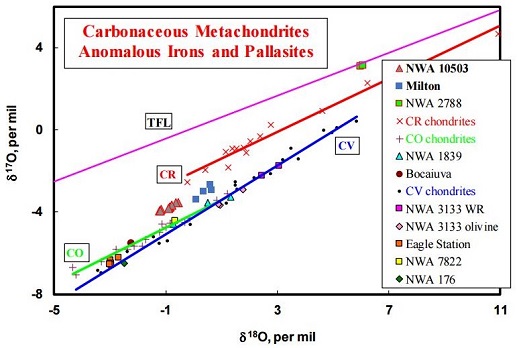
click on image for a magnified view Diagram credit: Irving et al., 79th MetSoc, #6461 (2016) In an effort to better resolve potential genetic relationship that might exist between Milton and the CV chondritesMeteorite class named after the Vigarano meteorite that fell in Italy in 1910. They have abundant large, well-defined rimless (?) chondrules of magnesium-rich olivine (~0.7 mm diameter; 40-65 vol. %), often surrounded by iron sulfide. They also contain 7-20 vol. % CAIs. The often dark-gray matrix is dominated by Fe-rich Click on Term to Read More, a Cr-isotopic analysis of olivine from the Milton pallasite was conducted by Sanborn et al. (2018). It is demonstrated on a coupled Δ17O vs. ε54Cr diagram (shown below) that Milton plots among the CV clan and plausibly shares a genetic relationship, but also that Eagle Station plots closer to the CK (or CO) chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More group. It could be inferred that both the CV and CK planetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More experienced a similar petrogenetic history in a similar isotopic reservoir of the nascent Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids.. Chromium vs. Oxygen IsotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More Plot
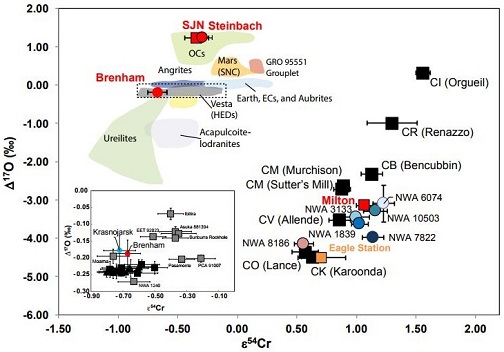
click on diagram for a magnified view Diagram credit: Sanborn et al., 49th LPSC, #1780 (2018) In an effort to further resolve differences between the CV and CK chondriteClass of carbonaceous chondrite named for the Karoonda meteorite that fell in Australia in 1930. They are more oxidized than all other carbonaceous chondrites and genetically distinct from CV chondrites. CK chondrites appear dark-gray or black due to a high percentage of Cr-rich magnetite dispersed in a matrix of dark Click on Term to Read More groups, Yin and Sanborn (2019) analyzed Cr isotopes in a significant number and broad range of meteorites. Their study included samples from each of the three CV subgroups (oxA, oxB, Red), anomalous CV3 chondrites, a C3-ungrouped, several CK members, and other potential CV-related meteorites including NWA 10503 and Milton (see top diagram below). It is demonstrated that the CV and CK meteorites are clearly resolved into two distinct isotopic reservoirs. In addition, it is shown by the ε54Cr values that NWA 10503 plots among the CV-related meteorites. A coupled Δ17O vs. ε54Cr diagram plotting all of the meteorites in their study is shown at the bottom below. Cr Isotope Weighted Average For CV and CK Chondrites
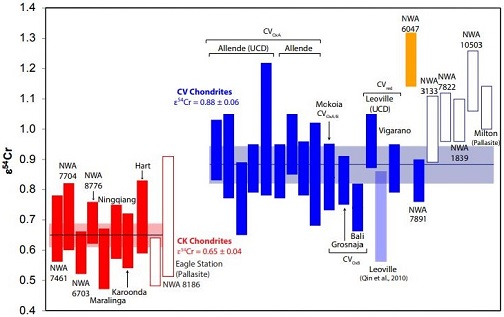
click on photo for a magnified view 17O vs. ε54Cr Diagram For CV and CK Chondrites
CK: orange shades; CV: green shades; Achondrites: open
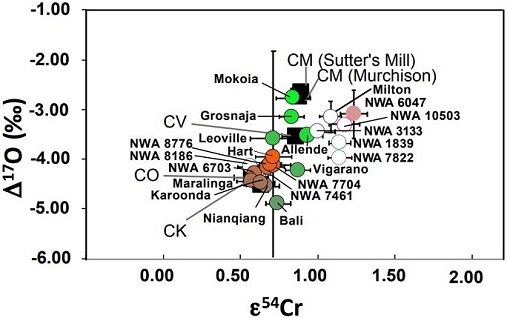
click on photo for a magnified view Diagrams credit: Yin and Sanborn et al., 50th LPSC, #3023 (2019)
In a study of two newly recovered ungrouped carbonaceous meteorites, the unequilibrated chondriteA chondrite with heterogeneous mineral compositions (e.g., olivine grains with differing FeO/(FeO+MgO) ratios. NWA 11961 and the dunitic brecciaWork in Progress ... A rock that is a mechanical mixture of different minerals and/or rock fragments (clasts). A breccia may also be distinguished by the origin of its clasts: (monomict breccia: monogenetic or monolithologic, and polymict breccia: polygenetic or polylithologic). The proportions of these fragments within the unbrecciated material Click on Term to Read More NWA 12264, Irving et al. (2019) further populated the O-isotopic trend line previously defined by NWA 10503 and the Milton pallasite; they termed this the ‘CX’ trend. However, Cr isotope data obtained for all of these meteorites have resolved both NWA 11961 and NWA 12264 as potential new carbonaceous parent bodies distinct from that of NWA 10503 and Milton, the latter previously considered possible members of the CV-clan (see diagrams below). ‘CX’ Oxygen Isotope Trend Line

click on photo for a magnified view O–Cr Diagram for ‘CX’ Trend Meteorites
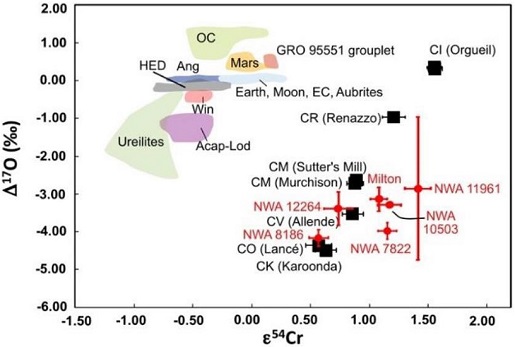
click on photo for a magnified view Diagrams credit: Irving et al., 50th LPSC, #2542 (2019)
Based on all of the data gathered so far, it could be concluded that the pallasites in our collections represent at least seven separate parent bodies: 1) main-group; 2) Eagle Station group; 3) Milton; 4) Choteau + Vermillion + Y-8451; 5) Zinder + NWA 1911; 6) NWA 10019; 7) LoV 263. In addition, several pallasites with anomalous silicates (e.g., Springwater) and anomalous metal (e.g., Glorieta Mountain) could possibly increase the number of unique parent bodies. The specimen of Milton shown above is a 40.1 g partial slice sectioned from an 85 g slice that was acquired from the owner of the main massLargest fragment of a meteorite, typically at the time of recovery. Meteorites are commonly cut, sliced or sometimes broken thus reducing the size of the main mass and the resulting largest specimen is called the "largest known mass". Click on Term to Read More, J. Piatek. The top two photos below show the cut face of a 500 g end section and the natural suface of a 677 g end section of Milton. The bottom photo shows a 2 cm-wide magnified image of an interior slice of Milton, courtesy of Dr. Laurence Garvey.


Photos courtesy of Dr. Jay Piatek

Photo courtesy of Dr. Laurence Garvie—Arizona State University







