Eagle Station
PallasiteOne of two main classes of stony-iron meteorite, the other being mesosiderites. Pallasites are igneous in nature and characterized by crystals of olivine, sometimes peridot (green gem quality clear olivine crystals), embedded in a matrix of Fe-Ni metal. The type specimen, weighing 680 kg, was found in the mountains near Click on Term to Read More, Eagle Station group
(possibly CK- or CO-related)
‘The Butterfly’
Found 1880
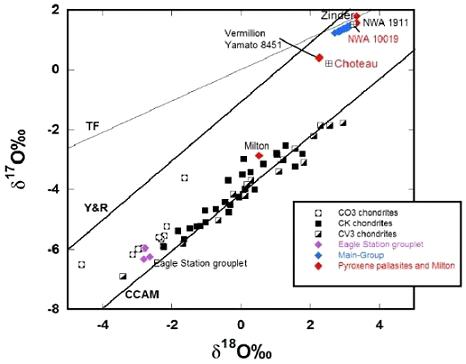
38° 37′ N., 84° 58′ W. A 36.5 kg mass was found about 0.75 miles from Eagle Station, Carroll County, Kentucky. Eagle Station has the highest fayalitePure* iron end-member (Fe2SiO4) of the olivine solid solution series and an important mineral in meteorites. When iron (Fe) is completely substituted by magnesium, it yields the the pure Mg-olivine end-member, forsterite (Mg2SiO4). The various Fe and Mg substitutions between these two end-members are described based on their forsteritic (Fo) Click on Term to Read More and Ni contents of all other pallasites, while Cold Bay and Itzawisis have nearly the same levels. In 2010 the Karavannoe pallasite was recognized as the the fourth member of this pallasite grouplet (Korochantsev et al., 2013), and in 2012 the fifth member, Oued Bourdim 001, was found—the grouplet has become a group. In consideration of these and other anomalous elemental ratios (e.g., high Ge/Ga, high Ni, and high Ir), along with unique O-isotopic ratios, these pallasites define a group distinct from the pallasites of the main-group, the pyroxene-bearing pallasites, and the other ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More pallasites Milton and Choteau. It is noteworthy however that Milton plots proximate to the O-isotopic trend line (CCAM slope) of the CV–CK-related meteorites and the Eagle Station pallasites (Korochantsev et al., 2013) (see diagram below). Also of interest is that the ‘Vermillion pallasite grouplet’ of pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More pallasites, comprising Vermillion, Choteau, and Y-8451, plots with the acapulcoite-lodranite clan on an oxygen three-isotope diagram.
Diagram credit: Gregory et al., 47th LPSC, #2393 (2016) Two very closely related silicated irons, Bocaiuva and NWA 176, also share many compositional similarities with the Eagle Station pallasites, and probably originated from similar chondritic material in the same region of the solar nebulaThe primitive gas and dust cloud around the Sun from which planetary materials formed.. Calculations indicate that the oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More CV chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More and the Eagle Station pallasites shared a similar composition of their parental metallic melts (Humayun and Weiss, 2011). Precise O-isotopic compositions for Eagle Station and Itzawisis (Δ17O = –5.22 [±0.05] ‰), along with bulk metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More Ni-isotopic compositions and siderophile elementLiterally, "iron-loving" element that tends to be concentrated in Fe-Ni metal rather than in silicate; these are Fe, Co, Ni, Mo, Re, Au, and PGE. These elements are relatively common in undifferentiated meteorites, and, in differentiated asteroids and planets, are found in the metal-rich cores and, consequently, extremely rare on abundances, were used support a genetic relationship among the Eagle Station pallasites, the Northwest Africa 176 silicated iron, and the CV/CK chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More (Ali et al., 2013, 2014). Of particular interest, the O- and Cr-isotopic signatures of Eagle Station have been used to establish a formation age of 4.557 (±0.6) b.y.

click on photo for a magnified view Diagrams credit: Sanborn and Yin, 50th LPSC, #1498 (2019)
In a more recent isotopic analysis of Eagle Station, Dey et al. (2019) obtained a Δ17O value of –4.93 ‰, which is more consistent with that of the CK chondrites. They employed newly obtained 17O and ε54Cr values for several irons, a pallasite, and their associated silicates/oxides to investigate i) if each iron/pallasite and the associated phases originated on a common parent body (i.e., an endogenous mixture of coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More and mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More vs. an exogenous mixture through impact), and ii) if any genetic connection exists between the irons/pallasite and other meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More groups (e.g., IAB with winonaites, IIE with H chondrites, and Eagle Station pallasites with CK chondrites). It was demonstrated on an O–Cr coupled diagram (UCD in diagram below) that the ε54Cr values for both the silicates and the oxide phase (chromite) in Eagle Station are identical and indicate an origin from a common reservoir—and thus, less consistent with an impact origin for the pallasite. Other results from their study can be found on the Caddo County and Miles pages. 17O vs. ε54Cr for Irons and Pallasites
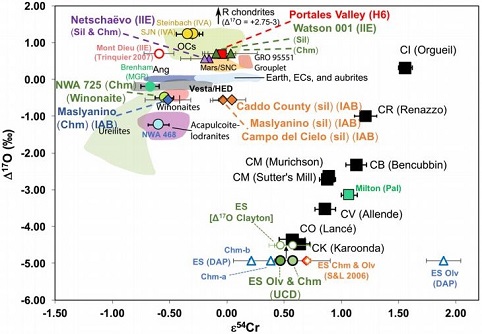
click on photo for a magnified view Diagrams credit: Dey et al., 50th LPSC, #2977 (2019)
Dispersed throughout the metal matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More of Eagle Station are angular, highly fragmented, cm-sized olivine crystals intermixed with sharp, irregular, sub-mm-sized olivine splinters. A multi-stage formation history has been proposed in which an initial impact generated enough heat to form a melt. After 20% fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More of this melt, both silicates and solid metal precipitated from the parental melt and accumulated, representing the material that would become the Eagle Station pallasites. A subsequent impact shattered the olivine and mobilized the metal, which flowed into existing cracks. Thereafter, deformational events produced shock forces which incorporated angular shards of olivine, schreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More, and chromite into melted troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More. Rounding of olivine crystals, once considered to be due to thermodynamic processes that minimize the capillary forces along the olivine–metal interface (Saiki et al., 2003), is now thought to occur primarily from resorption at high temperatures (above ~1250°C) in the presence of silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the melt (Boesenberg et al., 2012). The Eagle Station pallasites are confidently resolved from main-group pallasites in having higher Ni, Ge, Ir, Co, Re, Pt, and Cu contents, and lower As, AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More, and Ga in the metal. Karavannoe exhibits some anomalous elemental abundance ratios, possibly the result of very extensive terrestrial weathering. The Eagle Station pallasites also have higher Fe contents in the silicates compared to the main-group (Fa19–20 vs. Fa11–13); moreover, they have higher Sc and lower Mg and Mn. These elemental compositions, along with the O- and Cr-isotopic ratios, are similar to those in group IIF irons and the CO, CK, and oxidized CV carbonaceous chondrites, particularly Felix (CO3) and Tibooburra (CV3). In addition, Humayun et al. (2014) observed that many of the siderophile elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More abundances measured in Karavannoe and Eagle Station are a good match to the CV chondrites, and are indicative of formation in an oxidizingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More environment. Their studies suggest a sequencial formation for Karavannoe involving fractional crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More of a CV-like metallic melt that was more evolved than the Eagle Station metal. A scenario has been considered in which the Eagle Station pallasites were once a part of a large differentiated parent body that was collisionally disrupted. This is consistent with the finding of natural remanent magnetization in the CV chondrites, attesting to the existence of a core dynamo at the time these meteorites were formed (Weiss et al., 2010). Likewise, paleomagnetic studies conducted by Tarduno et al. (2014) revealed a strong natural remanent magnetization in tiny magnetic inclusions in Eagle Station olivines. Importantly, this remanent magnetization attests to the fact that the Eagle Station pallasite was not formed near the core-mantle boundary, because a rotating core dynamo would necessarily cease prior to any significant cooling of adjacent material; therefore, no remanent magnetization would exist. Application of the Hf–W isotopic chronometer to Eagle Station reveals that core formation occurred relatively late, ~10 m.y. after differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More of the likely HED parent body 4 VestaThird largest and fourth brightest asteroid; it was discovered in 1807 by Heinrich Olbers and named for the ancient Roman goddess of the hearth. 4 Vesta has a basaltic surface composition and an average density not much less than that of Mars. Evidently lava once flowed here indicating that the (Dauphas et al., 2005). It has been calculated that melting and core–mantle differentiation due to radiogenic heating should cease by ~7–8 m.y. (Sahijpal et al., 2007). This implies that heating of the Eagle Station asteroid continued until after all radiogenic 26Al and 60Fe was extinct, and that such late heating may have been generated through large impact events. Alternatively, the estimated initial Solar SystemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with ratio of 60Fe/56Fe may have been higher than previously considered leading to conditions conducive to a more prolonged core–mantle differentiation. The chemical composition of Karavannoe is consistent with formation after 60% fractional crystallization of metallic melt on the (CK?) parent body. Other features observed in this member of the Eagle Station group, such as troilite globules, and rounded olivines containing inclusionFragment of foreign (xeno-) material enclosed within the primary matrix of a rock or meteorite. Click on Term to Read More chains, are thought to record multiple severe and/or disruptive impact events. Employing three methods, Yang et al. (2010) determined the cooling rate of the Eagle Station pallasites at ~15 K/m.y., near the rate of the fastest cooled main-group pallasites. The CRE age of Eagle Station was determined by some to be 32 (±6) m.y., while others arrived at a value of 388 (±74) m.y. (Cook et al., 2010). Remarkably, multiple approaches conducted by Huber et al. (2010) resulted in a much longer CRE age of ~1 b.y. An estimate of the pre-atmospheric mass was calculated to be ~83.3 kg. The photo above shows a 0.69 g thin partial slice of Eagle Station. The photo below shows a large slice showing the typical distribution of silicate and metal in Eagle Station, courtesy of Sergey Vasiliev.

Photo courtesy of Sergey Vasiliev—SV-meteorites.com