NWA 2353
Meta-H
(Achondrite-ung in MetBull 89)
Purchased January 2004
no coordinates recorded A single meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More weighing 580 g was purchased by Astronomical Research Network in Erfoud, Morocco. The stone was analyzed and classified at Northern Arizona University (T. Bunch and J. Wittke) and was initially determined to be a completely recrystallized H chondriteOrdinary chondrites with a high content of free Ni-Fe metal (15-19 vol. %) and attracted easily to a magnet. Their main minerals are olivine (Fa16-20) and the orthopyroxene bronzite (Fs14.5-18.5), earning them their older name of bronzite chondrites. Chondrules average ~0.3 mm in diameter. Comparison of the reflectance spectra of Click on Term to Read More lacking any relict chondrules—an H7 chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More. The olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More Fa (17.9) and pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More Fs (15.6) values are consistent with the H group. Based on the completed O-isotope analysis, it is evident from the oxygen 3-isotope diagram that the ratios plot slightly outside of the main field of H chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, but still overlaps the H chondrite and IIE iron parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More.
| ORDINARY CHONDRITEWork in Progress Ordinary chondrites (OCs) are the largest meteorite clan, comprising approximately 87% of the global collection and 78% of all falls (Meteoritical Society database 2018)1. Meteorites & the Early Solar System: page 581 section 6.1 OC of type 5 or 6 with an apparent shock stage of S1, Click on Term to Read More COMPOSITIONS | ||
|---|---|---|
| Fa | Fs | |
| H | 16–20.4 | 14.5–18.1 |
| H/L | 19.5–21.8 | 17.2–21.2 |
| L | 22–26 | 18.7–22 |
| L/LL | 25.5–26.5 | — |
| LL | 26–33 | 22–26 |
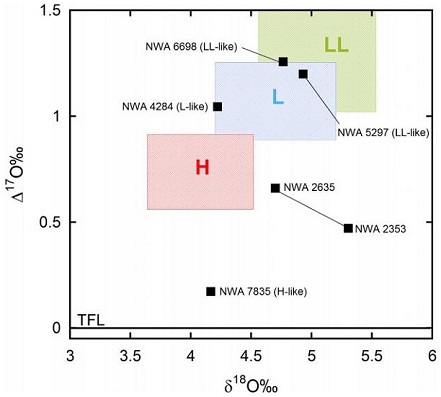
Diagram credit: Greenwood et al., Chemie der Erde, vol. 77, p. 24 (2017)
‘Melting and differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More of early-formed asteroids: The perspective from high precision oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More studies’
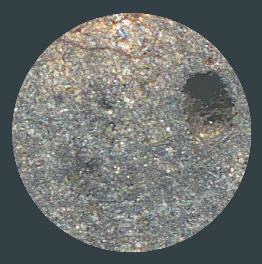
(open access: http://dx.doi.org/10.1016/j.chemer.2016.09.005) Northwest Africa 2353 shows evidence of having been very weakly shocked (stage S2, peak pressure of 5–10 GPa), and that it has undergone heavy oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More since its fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More (grade W3). The specimen of NWA 2353 shown above is a 2.8 g partial slice. The top photo below shows a photomicrograph of NWA 2353, while the middle photo shows the complete mass of this metachondriteTerm used to describe a metamorphosed chondrite. Also referred to as a type 7 chondrite. Metachondrites are texturally evolved rocks derived from chondritic precursors and some have been classified as primitive achondrites. Click on Term to Read More. The bottom photo shows a magnified portion of this specimen, exhibiting its recrystallized texture, a small melt vein, and a 1 mm-sized vug.
NWA 2353 viewed in partial crossed-polarized light (base width ~1 cm)
Photo courtesy of Dr. Ted Bunch—Northern Arizona University

Photo courtesy of Ken Regelman—Astronomical Research Network