Santa Clara
Iron, IVB, ataxiteWork in Progress Relatively rare variety of iron meteorite (designated type D) made almost entirely of taenite, a solid solution of Fe and 27 to 65% Ni. The Greek name means "without structure" and refers to the lack of a visible Widmanstätten pattern (spindles of kamacite are visible only microscopically). Click on Term to Read More
Found 1976
24° 28′ N., 103° 21′ W. A single 63 kg iron mass was found in Durango, Mexico and subsequently purchased by Arizona State University—Center for MeteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More Studies. Because it contains 17.9% nickel, it forms a macroscopically featureless surface structure of taeniteLess common than kamacite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Taenite, γ-(Fe,Ni), has 27-65 wt% Ni, and forms small crystals that appear as highly reflecting thin ribbons on the etched surface of a meteorite; the name derives from the Greek word for "ribbon." Click on Term to Read More. However, very fine, non-intersecting laths of kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More in a fine plessitic matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More can be seen on a microscopic scale. Santa Clara metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More contains little to no cloudy zone microstructure and lower Ni concentrations in taenite, which is evidence for a modest shock-reheating to above 400°C for a period of years, or 1000°C for a duration lasting only seconds (Goldstein et al., 2009).
- Following accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More of an ~220 km-diameter planetesimal ~0.1 (to ~0.5) m.y. after CAIs, and partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More ~0.2 m.y. later, the extraction and ascension of an early silicate partial melt removes ~90% of the radiogenic 26Al component from the interior. This results in the formation of a shallow magma oceanCompletely molten surfaces of terrestrial planets or moons that formed soon after accretion. Samples returned by the Apollo missions provide evidence of a lunar magma ocean, crystallization of which produced a stratified Moon with a low-density crust formed by accumulation of the mineral plagioclase overlying a higher density mantle of Click on Term to Read More (SMO) having a melt content of >50%. This silicate melt also sequesters a solid FeNi-metal component that is suspended within the SMO by convective forces. Over time, a separate, lower mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More ocean (MMO) is generated, metal particles gravitationally settle, and a proto-core evolves. The metal in this proto-core has a low 182W composition due to its early isolation from silicates, ~1.5 m.y. after CAIs, and exclusion of parental 182Hf. Therefore, the proto-core should reflect a Hf–W-based metal–silicate separation age significantly older than the observed age of ~2.9 (±0.6) m.y. after CAIs. This discrepancy in the timing of metal–silicate separation was resolved in their preferred model which invokes a second stage of differentiation. After an extended period of gradual cooling (~5.4 [3–11] m.y.), both the solid and the remaining liquid metal within the SMO, which had now become enriched in 182W through 182Hf decay, precipitate downward through the MMO and into an eventually fully molten core. This late-segregated metal then mixes with the low 182W, early-segregated metal thereby establishing a ‘combined’ Hf–W age for metal–silicate separation of ~2.9 (±0.6) m.y. after CAIs. Core crystallization is complete by ~30 m.y. after CAIs.
Schematic of the Evolution Scenario of the IVB Parent Body

click on photo for a magnified view
Full article in Journal of Geophysical Research: Planets (American Geophysical Union), vol. 123, #2, pp. 421-444, (2018)
‘Multistage Core Formation in PlanetesimalsHypothetical solid celestial body that accumulated during the last stages of accretion. These bodies, from ~1-100 km in size, formed in the early solar system by accretion of dust (rock) and ice (if present) in the central plane of the solar nebula. Most planetesimals accreted to planets, but many – Click on Term to Read More Revealed by Numerical Modeling and Hf‐W Chronometry of Iron Meteorites’
(https://doi.org/10.1002/2017JE005411) Yang et al. (2009) determined that the varied CRE history of the IVB group, as well as the wide range of cooling rates measured for its members, is consistent with a multiple breakup of the parent body and/or removal of the insulating mantle. Goldstein et al. (2010) found that Ni concentration profiles measured along the kamacite–taenite interface not only attest to one of the fastest cooling rates among iron groups, but also record a wide range of cooling rates in a similar manner to IVA and IIIAB irons. However, in contrast to IVA and IIIAB irons, which crystallized inwards following mantle removal, low-Ni IVB irons cooled slower than high-Ni IVB irons consistent with concentric crystallization from the center outwards, while temperatures were buffered by an insulating silicate mantle. That being said, to establish the wide variation in cooling rates that exists among IVB irons, the mantle would have to have been stripped prior to cooling below 600°C. Goldstein et al. (2010) and Yang et al. (2010) calculated that this impact event occurred while 25% of the outermost portion of the core was still molten, and that IVB irons were derived from the previously solidified portion within (see diagram below). Solidification of the innermost portion of the core proceeded after mantle removal (and possibly removal of the remaining liquid core), evidenced by the wide variation in cooling rates among IVB irons. The core in which IVB iron crystallization occurred was calculated to have been 110–170 km in diameter on a pre-disrupted asteroid that was 220–340 km in total diameter. The low-Ni IVB subgroup with the slowest cooling rate (200°C/m.y.) was located near the center of the core, while the high-Ni subgroup that crystallized late and had the fastest cooling rate (4700°C/m.y.) was located ~62 km from the center.
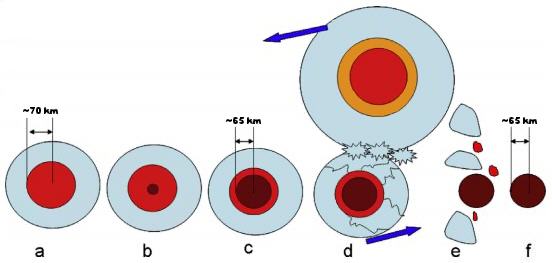
Diagram modified from Yang et al., GCA, vol. 74, p. 4503 (2010)
‘Thermal history and origin of the IVB iron meteorites and their parent body’
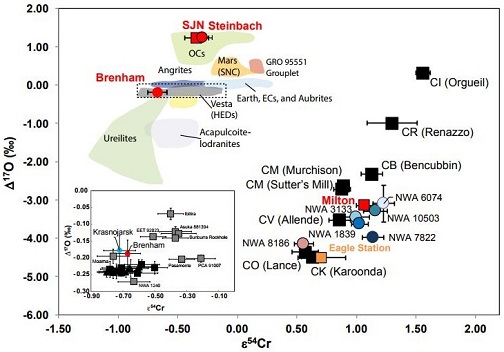
(https://doi.org/10.1016/j.gca.2010.04.011) It was reported by Campbell and Humayun (2005) that the depletion of moderately volatile elements in IVB irons is similar to that observed in the angriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More meteorites. In addition, the calculated length of time that the magnetic field persisted on the angriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More parent body (8 m.y.) is concordant with the crystallization period of the IVB irons. They speculated that the angrites might serve as a good representation of the hypothesized silicate portion of the IVB parent body. However, it was also suggested that the Fe/Mn ratio of the IVB silicate shell would probably have been high (~200) compared to the Earth (~60), and probably higher still than that of the angrite silicate shell (~120). With the advent of better investigative techniques, scientists have explored the possibility of a genetic relationship between IVB irons and other meteorite groups based on O-isotopic analyses. Utilizing chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More grains from IVB irons Warburton Range and Hoba, Corrigan et al. (2017) concluded that IVB irons are not genetically related to angrites, but that their respective parent bodies experienced similar petrogenetic histories. They also found that IVB irons share close similarities to the South Byron trio irons (Babb’s Mill [Troost’s], South Byron, and Inland Forts [ILD] 83500) and the Milton pallasiteOne of two main classes of stony-iron meteorite, the other being mesosiderites. Pallasites are igneous in nature and characterized by crystals of olivine, sometimes peridot (green gem quality clear olivine crystals), embedded in a matrix of Fe-Ni metal. The type specimen, weighing 680 kg, was found in the mountains near Click on Term to Read More (see top diagram below). Moreover, isotopic compositions (e.g., O, Mo, Ru, and W) and HSE abundances of the IVB irons and the South Byron trio–Milton grouping fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More within the range of the oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More CV–CK chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More (Hilton et al., 2018). In addition, Cr-isotopic analyses conducted by Sanborn et al. (2018) has provided further evidence for a genetic link between many of these disparate meteorites, possibly on a common, now disrupted CV parent body (see bottom diagram below).

Diagram credit: Corrigan et al., 48h LPSC, #2556 (2017) Chromium vs. OxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More IsotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More Plot
click on diagram for a magnified view Diagram credit: Sanborn et al., 49th LPSC, #1780 (2018) Rare silicaSilicon dioxide, SiO2. inclusions no larger than 25 µm in size have been reported in Santa Clara (and in IVB Warburton Range) associated with sulfide nodules (Teshima and Larimer, 1983). Despite the fact that this group of irons is considered to have formed from a volatile-depleted precursor under oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions (Campbell and Humayun, 2005), it was inferred that the presence of these inclusions in a nearly Si-depleted FeNi-metal host attests to formation under high-temperature, possibly late-stage reducingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions (but more oxidizing than for E chondrites). Further research is needed to better understand the nature of IVB parent body. To learn more about the relationship between the IVB irons and other iron chemical groups, click here. The specimen of Santa Clara shown above is a 10.0 g partial slice.







