Caddo County
Iron, IAB complex, Udei Station grouplet
Found 1987
35° 0′ N., 98° 20′ W. approx. A single mass weighing ~35 pounds along with fragments having a combined weight of ~5 pounds were found by a farmer while plowing. This is an unusual meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More in which chondritic and nonchondritic silicates are poorly mixed with the FeNi-metal host.
SilicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the inclusions typically contain olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More, pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More, plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More, chromian diopside, graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More, troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More, and phosphate. The mm-sized chromian diopside crystals within the silicate clasts have a pronounced green color. Na-rich plagioclase-diopside gabbros have been found in Caddo County, the first such basaltic material found associated with iron meteorites. Apart from this basaltic material, eucritesMost common type of achondrite meteorite and a member of the HED group. Eucrites are basalts composed primarily of pigeonite and anorthite (An60-98). Eucrites have been placed into three subgroups based on mineralogical and chemical differences. • Non-cumulate eucrites represent the upper crust that solidified on a magma ocean after Click on Term to Read More, angrites, and the ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More achondriteAn achondrite is a type of stony meteorite whose precursor was of chondritic origin and experienced metamorphic and igneous processes. They have a planetary or differentiated asteroidal origin where the chondritic parent body reached a sufficient size that through heating due to radioactive decay of 26Al (aluminum isotope) and gravitational Click on Term to Read More NWA 011 represent the only other asteroidal basalts known, while some basaltic plagioclase-enriched regions occur in two meteorites from the acapulcoite–lodraniteRare type of primitive achondrite named after the Lodran meteorite that fell in Pakistan in 1868. Initially, lodranites were grouped with the stony-iron meteorites because they contain silicates (olivine, orthopyroxene, and minor plagioclase) and Fe-Ni metal in nearly equal proportions. However, since discovery of the closely related acapulcoite group, lodranites Click on Term to Read More parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More. This coarse-grained, augite–albite-rich gabbroic material in Caddo County formed as an early-stage, localized partial melt from a chondritic parent body. Because of the high silicaSilicon dioxide, SiO2. content (59 wt%) of this material, along with its low olivine and orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More content, it represents the first asteroidal andesitic material positively identified.
Formation of IAB irons began with the partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More of a unique chondritic parent body, probably through a combination of both endogenous radiogenic heating (26Al decay) and impact events. Temperatures varied from as low as 950°C to as high as 1400°C, producing a range of metal–silicate lithologies. Migration of the partial melt into a S-rich coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More, or into numerous smaller pools distributed throughout the parent body, resulted in the segregation of silicates from metal–sulfide partial melts, probably resulting in the partial differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More of the asteroid. Based on the Hf–W systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with, this metal–silicate segregation began very early, within ~2.5 m.y. of the formation of CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More, and therefore silicate inclusions in IAB irons represent some of the oldest silicates available for study (Schulz et al., 2010).
It has been demonstrated through HSE data that the IAB complex subgroups were likely formed in distinct parental melt pools, possibly including a core component, with the observed fractionationConcentration or separation of one mineral, element, or isotope from an initially homogeneous system. Fractionation can occur as a mass-dependent or mass-independent process. Click on Term to Read More resulting primarily from crystal segregation rather than fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More processes (Wasson and Kallemeyn, 2002; Worsham et al., 2013). However, studies of the Mo isotopic compositions of representative meteorites from the IAB iron complex have demonstrated that both the sHL and sHH subgroups might derive from distinct parent bodies in separate nebular regions compared to the IAB complex irons (Dauphas et al., 2002; Ruzicka et al., 2006; Ruzicka, 2014; Worsham et al., 2014; Worsham and Walker, 2015; Worsham et al., 2017). Furthermore, in their studies of Mo isotopic compositions of IAB complex irons, Worsham and Walker (2015) reported anomalous negative µ95Mo values (where µ denotes parts in 106 deviation from terrestrial standards) for Caddo County, which if verified would suggest a formation on a unique parent body. However, HSE data for the Udei Station grouplet reported by Wasson and Kallemeyn (2002) indicates that these irons are closely related to the sLL subgroup, and a new study conducted by Worsham et al. (2016) coupling Pd vs. other HSEs supports this conclusion.
Utilizing the short-lived 182Hf–182W chronometer, corrected for neutronCharge-neutral hadron with a mass of 1.6748 x 10-27 kg, equivalent to 939.573 MeV, and an intrinsic angular momentum, or spin, of ½ (in units of h/2π). The neutron is a nucleon, one of the two basic constituents of all atomic nuclei (apart from 1H, which consists of a single Click on Term to Read More capture by 182W due to galactic cosmic raysHigh-energy subatomic particles mainly originating outside the Solar System that continuously bombard the Earth from all directions. They represent one of the few direct samples of matter from outside our solar system and travel through space at nearly the speed of light. These charged particles – positively charged protons or Click on Term to Read More, Hunt et al. (2018) derived the timing of metal–silicate separation of all genetically-related IAB irons (at least the MG and sLL subgroup [possibly also the sLM subgroup] along with Caddo County and Livingstone [Algarrabo duo]) to 6.0 (±0.8) m.y. after CAIs. Based on the constraints provided by the timing of metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More segregation, they modeled the early history of the 120(+)-km-diameter IAB parent body as outlined in the following diagram:
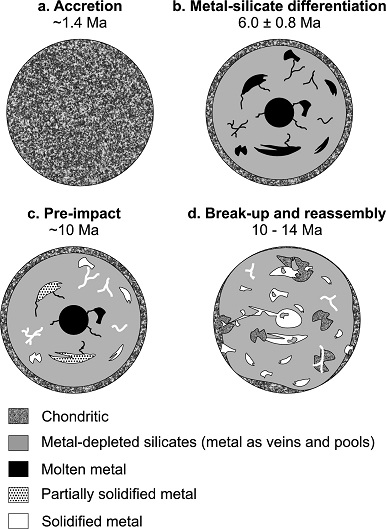
Diagram credit: Hunt et al., EPSL, vol. 482, pp. 497 (2018, open access link)
‘Late metal–silicate separation on the IAB parent asteroid: Constraints from combined W and Pt isotopes and thermal modelling’
(https://doi.org/10.1016/j.epsl.2017.11.034)’
Dey et al. (2019) employed 17O and ε54Cr values for several irons and their associated silicates/oxides to investigate i) if each iron and its associated phases originated on a common parent body (i.e., an endogenous mixture of core and mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More vs. an exogenous mixture through impact), and ii) if any genetic connection exists between the irons and other meteorite groups (e.g., IAB with winonaites, IIE with H chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, and Eagle Station pallasites with CK chondrites). Caddo County is one of three IAB irons employed in the study, and it was demonstrated on a coupled diagram that although the ε54Cr values for the iron component plot in the winonaitea partially differentiated asteroid that was disrupted just as it began to form an Fe core and a silicate-rich crust. This disrupting impact mixed silicates into molten Ni-Fe metal forming the silicated IAB irons, and mixed olivine-rich residues of partial melts into unmelted silicates, forming the winonaites. A few winonaites Click on Term to Read More field, values for the silicate component plot in a distinct region on an O–Cr coupled diagram (see diagram below). From these results they ascertained that the the IAB silicated irons formed through an impact-generated mixture comprising iron from a winonaite-related parent body and silicate from an unrelated and otherwise unsampled parent body. Incorporation of the silicates into the FeNi-metal host took place at a depth greater than 2 km, allowing time for a Thomson (Widmanstätten) structure to develop during a long cooling phase. Fractional crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More occurred in some large molten metal pools, followed by very slow cooling, to produce the broad range of features found in certain IAB meteorites (e.g., silicate-poor, graphite–troilite-rich inclusions and extremely high Ni contents). Other results from their study can be found on the Miles and Eagle Station pages.
17O vs. ε54Cr for Irons and Pallasites
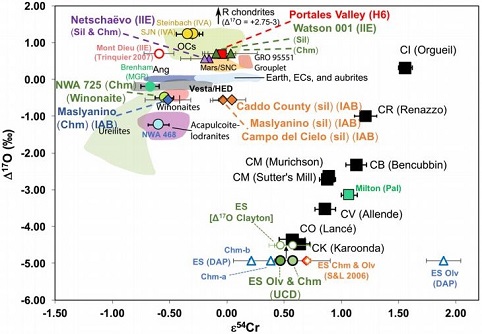
Diagrams credit: Dey et al., 50th LPSC, #2977 (2019)
Since the highest Ar–Ar age estimate for Landes would still make it younger than Caddo County, and since the cooling rate of metal is lower for Landes than that for Caddo County, it was inferred that Landes was the more deeply buried of the two source lithologies, both pre-disruption and post-reassembly of the IAB planetesimal (Vogel and Renne, 2008). Bogard et al, (2005) calculated the absolute I–Xe retention age relative to the Shallowater standard (4.5623 ±0.0004 b.y.) to be 4.5579 ±0.0001 b.y. (given that cooling was initiated 4.53 b.y. ago with an I–Xe closure temperature of 1100°C). In addition, they calculated the K–Ar closure age of Caddo County to be ~4.507 b.y.; a lower limit of 4.536 (±0.032) b.y. was calculated in a separate study (Vogel and Renne, 2006). Caddo County had a minimum pre-atmospheric diameter of ~40 cm, and a cosmic-ray exposure ageTime interval that a meteoroid was an independent body in space. In other words, the time between when a meteoroid was broken off its parent body and its arrival on Earth as a meteorite - also known simply as the "exposure age." It can be estimated from the observed effects Click on Term to Read More of only ~2 m.y., based on 3He, 21Ne, and 38Ar in metal; this CRE age is significantly lower than that of other IAB irons and the winonaites (Vogel and Leya, 2008).
Futher research on the petrogenetic history of the IAB silicated irons is presented by A. Ruzicka in Chemie der Erde, vol. 74, #1, pp 3–48 (2014); see also the Landes page. The specimen of Caddo County pictured above is a 19.6 g etched partial slice.







