Reid 013
Brachinite
Found 1991
30° 05′ S., 128° 55′ E. A mass of 330 g was found in the Nullarbor region of Western Australia. Its O-isotopic composition and bulk chemistry matched that of four previously identified olivine-rich achondrites. Interestingly, another brachinite, Reid 027, was recovered in the same region as Reid 013 (~59 km away), but it has been argued that it is unpaired to Reid 013 in light of grain size and plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More compositional differences. An ~517 g stone, designated NovaStar that, over a period of a few days, becomes 103 to 104 times brighter than it was previously. Novae are observed about 10-15 times per year in the Milky Way. Click on Term to Read More 003 (syn. Window Butte), was presumably found in Western Australia during the same year and is considered to be likely paired with Reid 013 (M. Prinz, AMNH; Lewis and Clark, ASU).
Reid 013 is composed primarily of olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More (<90 vol%) forming 120° triple junctions, minor clinopyroxene (~6 vol%) and chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More (~1 vol%), along with trace amounts of orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More, FeNi-metal, and sulfides (Hasegawa and Mikouchi, 2016). Brachinites are resolved into a small, unique, but diverse group, which was initially identified in 1983 with the discovery of the Brachina meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More. Interestingly, on an oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More three-isotope diagram Brachina plots well away from the other known brachinites and is close to the winonaitea partially differentiated asteroid that was disrupted just as it began to form an Fe core and a silicate-rich crust. This disrupting impact mixed silicates into molten Ni-Fe metal forming the silicated IAB irons, and mixed olivine-rich residues of partial melts into unmelted silicates, forming the winonaites. A few winonaites Click on Term to Read More field (Greenwood et al., 2007). Another feature of Brachina that is contrary to other brachinite members is its high abundance of plagioclase, measured to be 9.9 vol%—other brachinites contain none or only trace amounts (Mittlefehldt et al., 1998).
Brachinites exhibit significant O-isotopic heterogeneity with a dispersion of 0.17‰, a value which lies between that of HEDs (0.12‰) on one hand, and both the winonaite group (0.3‰) and the acapulcoite–lodraniteRare type of primitive achondrite named after the Lodran meteorite that fell in Pakistan in 1868. Initially, lodranites were grouped with the stony-iron meteorites because they contain silicates (olivine, orthopyroxene, and minor plagioclase) and Fe-Ni metal in nearly equal proportions. However, since discovery of the closely related acapulcoite group, lodranites Click on Term to Read More clan (0.75‰) on the other (Rumble III et al., 2008). It is inferred that the brachinites either formed on a single heterogeneous parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More, or on multiple bodies that underwent similar petrogenetic processes (Gardner-Vandy et al., 2012). This relatively high O-isotopic heterogeneity of brachinites has persuaded some that the meteorites should be considered primitive achondrites (Nehru et al. , 1992). However, other investigators have argued that brachinites are differentiated, ultramaficTerm used for silicate minerals with cations predominantly Mg and/or Fe. Mafic minerals are dominated by plagioclase and pyroxene, and also contain smaller amounts of olivine. Click on Term to Read More achondrites representing cumulates (Mittlefehldt et al., 2003), or partial melt residues (Gardner-Vandy et al., 2013) of high-temperature igneous melts.
Brachinites represent products of metamorphism and oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More of chondritic material, some likely formed as a partial melt residue (restite) and others as a cumulateIgneous rock composed of crystals that have grown and accumulated (often by gravitational settling) in a cooling magma chamber. Click on Term to Read More. Some brachinites and brachinite-like meteorites, including Reid 013, Hughes 026, NWA 7388, and NWA 7605, contain secondary reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More features possibly formed during parent body metasomatism by methane (Irving et al., 2013). Brachinites such as ALH 84025 and EET 99402/07 exhibit several features that are consistent with a cumulate origin: 1) high olivine CaO content consistent with a high-temperature, igneous origin; 2) cumulate-textured plagioclase grains enclosing olivine grains; 3) crystallographic preferred orientation (CPO) comprising foliation [planar alignment] and lineation [much longer in one dimension than in the other two] of olivine; 4) elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More patterns inconsistent with the removal of a metal–sulfide melt; 5) lack of tectonic strain features; 6) mineralogy exhibiting igneous textures rather than metamorphicRocks that have recrystallized in a solid state due to changes in temperature, pressure, and chemical environment. Click on Term to Read More recrystallization; and 7) REEOften abbreviated as “REE”, these 16 elements include (preceded by their atomic numbers): 21 scandium (Sc), 39 Yttrium (Y) and the 14 elements that comprise the lanthanides excluding 61 Promethium, an extremely rare and radioactive element. These elements show closely related geochemical behaviors associated with their filled 4f atomic orbital. Click on Term to Read More patterns and elemental ratios more consistent with igneous rocks containing cumulate plagioclase. A petrographic analysis of Reid 013 conducted by Hasegawa and Mikouchi (2016) revealed no obvious preferred orientation of olivine crystals, which is consistent with a partial melt residue rather than a cumulus origin. However, in another investigation of olivine CPO in brachinite EET 99402/07, Hasegawa et al. (2017) found a CPO pattern (preferential alignment along the b axis) similar to that observed in Reid 013 and the brachinite-like MIL 090206 pairing group, one which is indicative of high-degree melting and olivine accumulation; further study is needed to distinguish between a CPO pattern associated with compaction of residual grains and one associated with accumulation from a melt.
Alternatively, studies have demonstrated that partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More and melt removal of a chondritic source similar to R chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More at an oxygen fugacityUsed to express the idealized partial pressure of a gas, in this case oxygen, in a nonideal mixture. Oxygen fugacity (ƒO2) is a measure of the partial pressure of gaseous oxygen that is available to react in a particular environment (e.g. protoplanetary disk, Earth's magma, an asteroid's regolith, etc.) and Click on Term to Read More of IW–1 could lead to the formation of FeO-rich brachinites (Gardner-Vandy and Lauretta, 2011; Gardner-Vandy et al., 2012, 2013). It has been proposed that the brachinites could be divided into two subgroups (Nehru, et al., 1996): those which are near-chondritic and undepleted (UBRA) (e.g., Brachina and Reid 013), and those depleted in a basaltic component (DBRA) (e.g., ALH 84025, Eagles Nest, and Hughes 026). The primitive acapulcoite–lodranite clan has a similar scenario with an undepleted member (ACA) and a depleted member (LOD), although this group is reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More instead of oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More. Moreover, the loss of an Fe–Ni–S partial melt in the lodranites is not analagous to the siderophile–chalcophile element pattern seen in brachinites. Further evidence for a formation of brachinites as a partial melt residue is provided on the NWA 3151 page. Ultimately, brachinites were thermally metamorphosed to type 6 or 7 creating the highly equilibrated mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More compositions and recrystallized textures that are observed. A thermal event at ~4.13 b.y. ago is revealed through K–Ar isotopic chronometry.
Brachinites have trace element compositions similar to those of winonaites and silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the inclusions in IAB-IIICD irons: a high ratio of refractory metals to nonrefractory metals (Ir/Au), depletion of chalcophiles (through loss of Fe–Ni–S melt), and high volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). abundances (Zn). If the highly oxidized brachinites were to undergo redoxOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More exchange processes, the resulting chemistry would be very similar to that of the pyroxene-rich winonaites. On an oxygen 3-isotope diagram, the brachinites overlap parts of the winonaite–IAB complex field, as well as parts of the HED and angriteType of evolved achondrite meteorite that represent some of the earliest stages of asteroidal differentiation and magmatism in our solar system. Angrites are named for the Angra dos Reis meteorite, which fell in Rio de Janeiro, Brazil, in early 1869. They are basaltic (mafic) rocks, often containing porous areas, and Click on Term to Read More fields. Moreover, O-isotopes and mineralogy for several additional olivine-rich achondrites, including Zag (b), Divnoe, and NWA 4042, plot very close to the brachinite field, and therefore many or all of these meteorites might be members of the brachinite group, or could reflect formation by analogous processes on different parent bodies. In a like manner, the acapulcoite–lodranite clan also falls on the same O-mixing line, suggesting that each of these diverse groups may be related to a common chondritic precursor through variable redox processes.
In two recent consortia, analyses of the unique Antarctic alkalic igneous meteorite GRA 06128/9 was conducted. It was suggested that this thermally metamorphosed meteorite containing cumulate sodic plagioclase may represent moderate degrees of fractional melting (13–30%) at intermediate temperatures (<1200°C) on an L-like chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More parent body having chondritic abundances of moderately-volatile elements (Shearer et al., 2009). This alkali-rich partial melt phase was subsequently extracted under volatility-enhanced (possibly water) conditions, after incorporation of a low-temperature Fe–Ni–S melt phase. GRA 06128/9 has Δ17O-isotopic values, Fe/Mn ratios, and olivine Ni contents that are similar to those of brachinites (and with similar trends to those of angrites, pallasites, mesosiderites, and VestaThird largest and fourth brightest asteroid; it was discovered in 1807 by Heinrich Olbers and named for the ancient Roman goddess of the hearth. 4 Vesta has a basaltic surface composition and an average density not much less than that of Mars. Evidently lava once flowed here indicating that the), although its δ18O values are higher than those of brachinites and other meteorite groups. It also has major, minor, and trace element chemistry similar to brachinites, and it was formed under similar redox conditions.
Laboratory melting experiments conducted by Gardner-Vandy et al. (2013) have demonstrated that an FeO-rich (oxidized) R chondrite-like precursor asteroid can undergo significant partial melting (14–31% at ~1250°C) and melt removal to produce a brachinite-like residue, and possibly also a low degree partial melting (<10% at <1250°C) and melt removal to produce a complementary evolved melt having a composition like that of the brachinite-related GRA 06128/9. Sosa et al. (2017) employed multiple modeling techniques and conducted further melting experiments utilizing R4 chondrite LAP 03639. Their results demonstrate that an R chondrite-like precursor asteroid can undergo low-degree partial melting (~16–20%) at 1140°C at an oxygen fugacity of ~IW to produce a brachinite-like residue and a complementary evolved melt with a composition like that of GRA 06128/9.
Additional experimental data and modeling results attained by Lunning et al. (2017) have further constrained the conditions of formation for GRA 06128/9. Their investigation indicates that both equilibriumTerm used to describe physical or chemical stasis. Physical equilibrium may be divided into two types: static and dynamic. Static equilibrium occurs when the components of forces and torques acting in one direction are balanced by components of forces and torques acting in the opposite direction. A system in static Click on Term to Read More and non-equilibrium partial melting (the latter condition corresponding to lower temperatures and degrees of melting) on an oxidized parent body similar to R chondrites, in which 14–22% melt is generated at a temperature of 1120–1140°C and a redox state of IW–IW+1, reproduces most closely the whole rock composition of the GRA 06128/9 meteorite. The authors also posit that unsampled lithologies containing higher silicaSilicon dioxide, SiO2. abundances may have been produced on the GRA 06128/9 (or the brachinite) parent body, in association with very low degrees of non-equilibrium partial melting. These potential lithologies might be akin to the Almahata Sitta trachyandesite samples MS-MU-011/035, which are thought to represent the primary crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More of the ureilite parent body.
A very ancient crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More age of the GRA achondrites based on Sm–Nd and Al–Mg systematics is 4.5649 (±0.0002) b.y., consistent with that of Brachina (Mn–Cr age of 4.5648 [± 0.0005] b.y.; Dunlap et al., 2016) and other brachinites, and consistent with ancient magmatism and crustal formation on the brachinite parent body just 2.65 m.y. after CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More formation (Wimpenny et al., 2011). Dating single feldsparAn alumino-silicate mineral containing a solid solution of calcium, sodium and potassium. Over half the Earth’s crust is composed of feldspars and due to their abundance, feldspars are used in the classification of igneous rocks. A more complete explanation can be found on the feldspar group page. Click on Term to Read More grains in GRA 06128 gave an age of 4.34 (±0.02) b.y. (Lindsay et al., 2011). A model developed by Senshu and Usui (2011) predicts that formation occurred at a depth of <12 km on a parent body 36–100 km in diameter. As with brachinites and the other inner Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. objects, the Sm–Nd age of the GRA meteorite was reset ~3.4 b.y. ago, close to the Late Heavy BombardmentPeriod between ~4.0 to 3.8 Ga ago when the Moon and other objects in the Solar System were pounded heavily by wayward asteroids. The evidence for the Late Heavy Bombardment (LHB) includes the lunar maria basins and similar structures elsewhere, such as the Caloris Basin on Mercury and the great Click on Term to Read More period. Some investigators argue that GRA 06128/9 is most likely a member of the brachinite group based on an examination of the metal-sulfide segregation processes and the observed highly siderophile elementLiterally, "iron-loving" element that tends to be concentrated in Fe-Ni metal rather than in silicate; these are Fe, Co, Ni, Mo, Re, Au, and PGE. These elements are relatively common in undifferentiated meteorites, and, in differentiated asteroids and planets, are found in the metal-rich cores and, consequently, extremely rare on (HSE) abundances (Zeigler et al., 2008; Day et al., 2012).
Many of the known brachinites have disparate cosmic-ray exposure ages, indicating that they represent numerous separate ejection events. According to a study by Patzer et al. (2003), the CRE ages of EET 99402/407, Hughes 026, and Eagles Nest form a cluster at ~48 m.y., and those of Reid 013 and ALH 84025 coincide at ~10 m.y. In a separate study by Ma et al. (2003), the cosmogenic nuclideA nuclear species characterized by Z protons and N neutrons. Click on Term to Read More calculations establish a range of CRE ages from 4 m.y. for Brachina to ~25.5 m.y. for Eagles Nest. From their noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More analyses of 15 brachinite and brachinite-like meteorites, together with the literature values for seven others, Beard et al. (2018) identified three potential CRE age clusters. The youngest cluster reflects a possible ejection event that occurred ~10.5 (±1.1) m.y. ago, comprising the two brachinites Reid 013 and ALH 84025, and the two brachinite-like meteorites NWA 1500 and NWA 4518. Importantly, two of these CRE age clusters include both brachinite and brachinite-like meteorites, which attests to a common parent body for all of these meteorites (see diagram below). 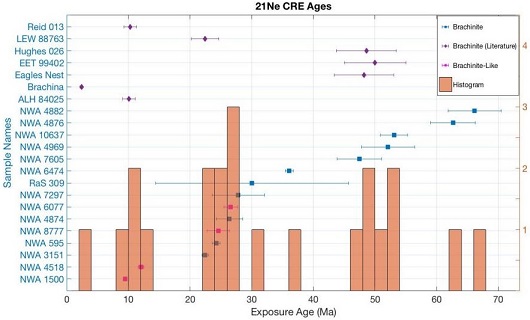
click on image for a magnified view
Diagram credit: Beard et al., 81st MetSoc, #6170 (2018) As demonstrated by Sanborn et al. (2014), a coupled Δ17O vs. ε54Cr diagram is one of the best diagnostic tools for determining genetic relationships between meteorites. Moreover, Sanborn et al. (2015) demonstrated that ε54Cr values are not affected by aqueous alteration. The diagram below includes the brachinites Brachina, NWA 3151, and the GRA 06128/9 paired stones, and it is apparent that they all plot very near each other in Δ17O–ε54Cr space, consistent with formation in a common isotopic reservoir. 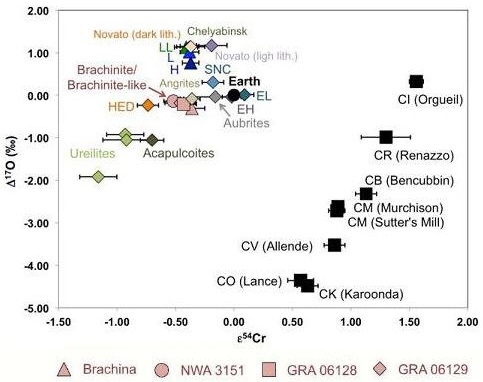
Diagram credit: Sanborn and Yin, 46th LPSC, #2241 (2015) Current research to determine the olivine compositions of asteroids has established a strong link between brachinites and the A-type asteroids 289 Nenetta and 246 Asporina (Sunshine et al., 2007). While there has been some speculation that Venus may possibly be the source for the brachinites (Shearer et al., 2008), others have recognized the isotopic, geochemical, and mineralogical similarities that exist between GRA 06128/9 and the IAB complex irons such as Caddo County and consider this iron asteroid to be a plausible source (Nyquist et al., 2009). However, a comparison between brachinites and IAB irons utilizing nucleosynthetic isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More anomalies (Mo systematics) shows them to be unrelated. 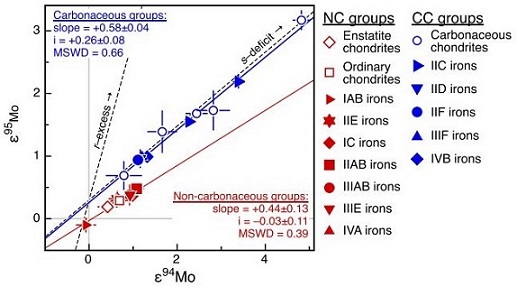
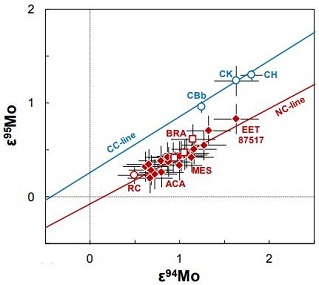
Diagrams credit:
(left) Kruijer et al., PNAS, vol. 114, #26, p. 6713 (2017), ‘Age of Jupiter inferred from the distinct genetics and formation times of meteorites’ (http://dx.doi.org/10.1073/pnas.1704461114)
(right) Budde et al., 49th LPSC, #2353 (2018) Reid 013 is among the least weathered brachinites discovered to date. The photo above shows a 1.3 g slice of Reid 013, with the reverse shown below.








