NWA 1463
ChondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More (type 5+)
previously Winonaitea partially differentiated asteroid that was disrupted just as it began to form an Fe core and a silicate-rich crust. This disrupting impact mixed silicates into molten Ni-Fe metal forming the silicated IAB irons, and mixed olivine-rich residues of partial melts into unmelted silicates, forming the winonaites. A few winonaites Click on Term to Read More (primitive) or ‘W Chondrite’
Purchased November 27, 2000
no coordinates recorded Three fragments having a combined weight of 1,001 g were found and subsequently sold in Erfoud, Morocco to Canadian collector D. Gregory. This meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More was analyzed and classified through a collaboration with UCLA and Washington University in St. Louis, and it was determined to be a primitive winonaite. A 23 g specimen of NWA 1463 is curated by UCLA, while the 975 g type specimen is on deposit with the Royal Ontario Museum.
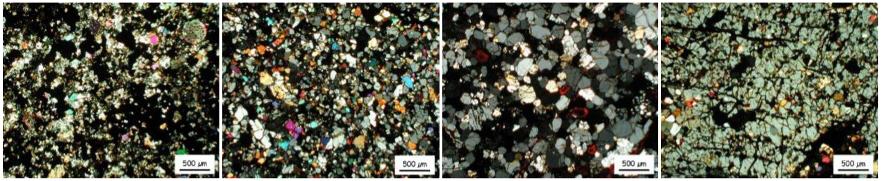
Textural comparison of four winonaites, L to R: NWA 1463 (with relict chondruleRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More), Winona, Tierra Blanca, HaH 193
Image credit: Floss et al., MAPS, vol. 43, #4, p. 660 (2008)
‘Evolution of the winonaite parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More: Clues from silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the mineral trace elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More distributions’
(http://dx.doi.org/10.1111/j.1945-5100.2008.tb00676.x) The O-isotope composition of NWA 1463 plots on an extension of the winonaite trend line. However, the high abundance of FeNi-metal and troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More, as well as the absence of metallic and sulfide veining, attests to a lower equilibration temperature than that of other winonaites.
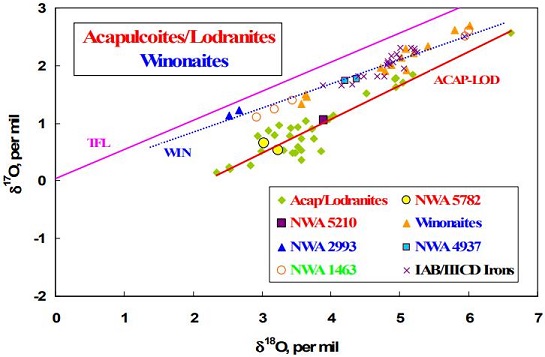
Diagram credit: Bunch et al., 41st LPSC, #1281 (2010) Based on similar silicate textures, reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More mineral chemistry, and O-isotopes, it is presumed that the winonaites and the IAB complex irons originated on a common parent body. Because of its highly primitive nature, NWA 1463 might closely resemble the chondritic precursor material of the typical winonaites and silicate inclusions in IAB complex irons. In a similar way, it was determined by Hidaka et al. (2015) that FeNi-metal in the winonaite Y-8005 retains a near chondritic composition likely representative of the precursor material of the parent body. Utilizing a Ge/Ni vs. AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More/Ni coupled diagram, they found that metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More in this winonaite plots in the field of the sLL subgroup of the IAB complex irons. In view of these findings, Hidaka et al. (2015) suggest that the sLL subgroup rather than the main group of the IAB complex represents the primitive metal of the IAB–winonaite parent body, with the main group possibly representing a partial melt of the sLL subgroup. In a subsequent analysis of the IAB iron complex, Worsham et al. (2017) demonstrated that the Mo isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More data for the two winonaites they studied, Winona and HaH 193, also attest to a common parent body for winonaites and the MG/sLL irons. The winonaite NWA 1463 does not fit into the scheme that has commonly been used to define the primitive achondriteAn achondrite is a type of stony meteorite whose precursor was of chondritic origin and experienced metamorphic and igneous processes. They have a planetary or differentiated asteroidal origin where the chondritic parent body reached a sufficient size that through heating due to radioactive decay of 26Al (aluminum isotope) and gravitational Click on Term to Read More group, and it could be instrumental in the future in redefining the metamorphicRocks that have recrystallized in a solid state due to changes in temperature, pressure, and chemical environment. Click on Term to Read More progression of chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More to primitive achondrites. To that end, Irving et al. (2005) described this meteorite as possibly representing the regolithMixture of unconsolidated rocky fragments, soil, dust and other fine granular particles blanketing the surface of a body lacking an atmosphere. Regolith is the product of "gardening" by repeated meteorite impacts, and thermal processes (such as repeated heating and cooling cycles). Click on Term to Read More of the winonaite parent body. Furthermore, they argued that the occurrence of distinct chondrules precludes the use of the term achondrite to describe this meteorite, and suggest that the term metachondriteTerm used to describe a metamorphosed chondrite. Also referred to as a type 7 chondrite. Metachondrites are texturally evolved rocks derived from chondritic precursors and some have been classified as primitive achondrites. Click on Term to Read More or ‘W chondrite’ would be more appropriate to describe this texturally-evolved meteorite pairing group (Irving et al., 2005; Irving and Rumble III, 69th MetSoc, #5288 [2006]). Northwest Africa 1463 contains certain features that are unique compared to most other winonaites, and while it is plausible that this meteorite may represent the winonaite precursor material, it has been conjectured that it may instead have originated on a separate parent body (Floss et al., 2008). These unusual features include the lowest degree of metamorphism of all winonaites, an anomalous chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More composition, an anomalous O-isotopic composition, a lack of graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More, the presence of merrillite rather than apatite, and an abundance of incompatible trace elements intermediate to other winonaites. The variability in Ar–Ar ages obtained for some winonaites indicates they may have been excavated from different depths over an extended period of time (Scott et al., 2014). Furthermore, cooling rates standardized at ~500°C were determined for a number of winonaites and IAB irons, and the results demonstrate that a wide continuum exists:
- Pontlyfni—1,000–10,000°C/m.y.
- Winona—200°C/m.y.
- NWA 1463—100°C/m.y.
- Fortuna and QUE 94535—30°C/m.y.
- IAB irons—10–25°C/m.y.
Based on the oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More isotope data obtained by Hunt et al. (2012) for silicate inclusions in IAB irons, along with the observed volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). element depletions, it can be inferred that the winonaite precursor likely had a volatile-depleted carbonaceous chondrite-like composition. From results of their trace element analyses of a broad sampling of winonaites, Hunt et al. (2017) recognized that CM chondrites represent the closest match; however, the important differences that exist indicate that the precursor to winonaites is unlike any meteorite class currently known. In another study employing Mo and W isotope data (e.g., Kruijer et al., 2017) demonstrate that the IAB complex irons, and thus the genetically-related winonaites, accreted in the non-carbonaceous reservoir (see the Appendix, Part III for further details). Preliminary data based on Al–Mg chronometry show that NWA 725 was formed ~1.4 m.y. after CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More. This age is consistent with Hf–W ages in the range of 1.5–5 m.y. that were calculated for more highly metamorphosed winonaites (Hidaka et al., 2014).
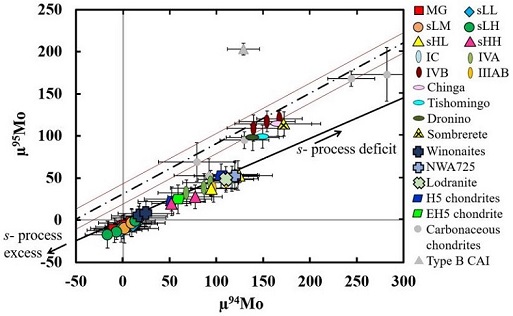
New analyses were conducted by Worsham et al. (2017) for IAB complex irons, along with two winonaites (Winona and HaH 193), a lodraniteRare type of primitive achondrite named after the Lodran meteorite that fell in Pakistan in 1868. Initially, lodranites were grouped with the stony-iron meteorites because they contain silicates (olivine, orthopyroxene, and minor plagioclase) and Fe-Ni metal in nearly equal proportions. However, since discovery of the closely related acapulcoite group, lodranites Click on Term to Read More (GRA 95209), the primitive achondrite NWA 725, and other selected meteorite groups. Employing precise Mo, W, and Os isotope data along with HSE and other literature data, they ascertained that the IAB complex irons represent at least three distinct parent bodies and at least three impact-generated metal–silicate segregation events (see top schematic diagram below). Moreover, they ascertained that the Mo isotope data, as well as the chemical and mineralogical data, attest to a common parent body for the winonaites and the MG/sLL irons. Importantly, they demonstrated that the Mo isotope values of NWA 725 do not plot with the IAB MG/sLL/winonaites, and that the values are all higher than those of the lodranite in their study. Notably, the Mo isotope values of NWA 725 plot within the field of the magmatic sHL and sHH irons, which are not genetically related to the other IAB parent bodies (see bottom diagram below). Oxygen isotope data for the sHL and sHH irons could help resolve whether any potential genetic relationship exists with the NWA 725 pairing group.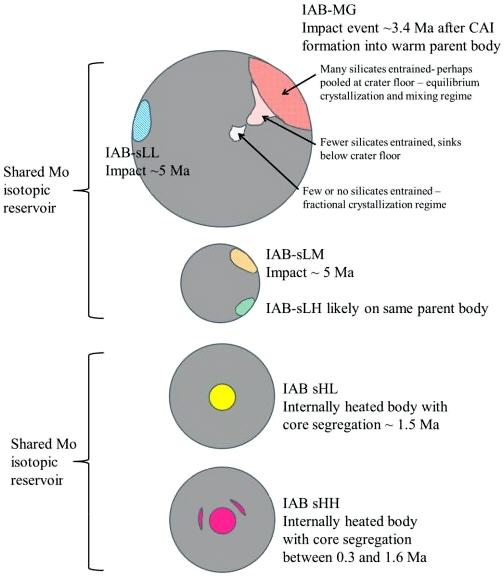
CRE-corrected Mo Isotopic Compositions of Meteorite Groups
(µ notation denotes deviation from terrestrial standards in parts per million)
click on photo for a magnified view
‘Characterizing cosmochemical materials with genetic affinities to the Earth: Genetic and chronological diversity within the IAB iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More complex’
(https://doi.org/10.1016/j.epsl.2017.02.044) There is convincing evidence that NWA 1463 is paired with NWA 725, NWA 1052, NWA 1054, and NWA 1058 (Irving and Rumble III, 2006); a further pairing was found in 2007 and designated NWA 4835 (T. Bunch, NAU). Further details about this pairing group can be found on the NWA 725 page. Shown above are two views of a 0.12 g cut fragment of NWA 1463 which show its chondritic texture and relatively fresh fusion crustMelted exterior of a meteorite that forms when it passes through Earth’s atmosphere. Friction with the air will raise a meteorite’s surface temperature upwards of 4800 K (8180 °F) and will melt (ablate) the surface minerals and flow backwards over the surface as shown in the Lafayette meteorite photograph below. Click on Term to Read More. This specimen was kindly provided by Dr. David Gregory.







