Zag
H3–6
Fell August 4 or 5, 1998
~27° 20′ N., 9° 20′ W. This fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More was reportedly observed from a mountain near Zag, Morocco. A large quantity was imported by meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More dealer A. Lang under the name Kem Kem, and about 175 kg has been distributed under the names Tan-Tan, Sagd, and Zag, with Zag becoming the official name. Zag is a gas-rich regolithMixture of unconsolidated rocky fragments, soil, dust and other fine granular particles blanketing the surface of a body lacking an atmosphere. Regolith is the product of "gardening" by repeated meteorite impacts, and thermal processes (such as repeated heating and cooling cycles). Click on Term to Read More brecciaWork in Progress ... A rock that is a mechanical mixture of different minerals and/or rock fragments (clasts). A breccia may also be distinguished by the origin of its clasts: (monomict breccia: monogenetic or monolithologic, and polymict breccia: polygenetic or polylithologic). The proportions of these fragments within the unbrecciated material Click on Term to Read More composed of both light and dark clasts (H6) within a gray clastic matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More (H3–4) containing chondrulesRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More. Components in Zag exhibit shock features ranging from S2 to S4, with bubbles of salty fluid inclusions entrained within xenolithic halite (NaCl) crystals found only in the H3–4 matrix component (Zolensky et al., 2013). Halite grains have previously only been found in the Monahans (1998) H5 chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, while both halite and sylvite (µm- to sub-µm-sized) have been observed on the external surface of numerous particles returned from the S-type asteroid Itokawa by the Hayabusa spacecraft (Noguchi et al., 2014).
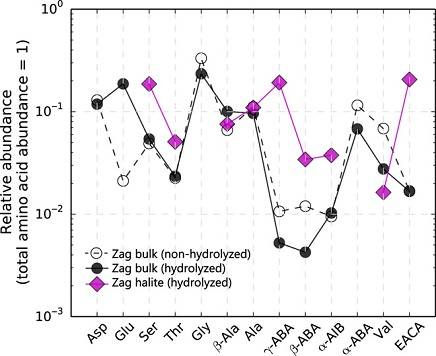
Diagram credit: Chan et al., Science Advances, vol. 4, #1, p. 6 (2018 open access)
‘Organic matter in extraterrestrial water-bearing salt crystals’
https://dx.doi.org/10.1126/sciadv.aao3521 Notably, a carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More clastA mineral or rock fragment embedded in another rock. Click on Term to Read More first identified in a Zag sample by O. Richard Norton has a mineralogy and an O-isotopic composition consistent with the CI chondriteRare meteorite class named after the Ivuna meteorite that fell in Tanzania in 1938. They are among the most primitive, friable (crumbly), and interesting of all meteorites, having undergone extensive aqueous alteration. They lack chondrules and CAIs as a result of this alteration, but contain up to 20% water, as Click on Term to Read More group, although it plots along an extension of the CI group with a high Δ17O of +1.41 to +1.49 (Zolensky et al., 2003; Zolensky et al., 2016). This fine-grained CI-like clast is primarily composed of phyllosilicatesClass of hydroxyl-bearing silicate minerals with a sheet-like structure. They result from aqueous alteration are dominantly serpentine and smectite in meteorites; found in the matrixes of carbonaceous chondrites. Phyllosilicates consist of repeating sequences of sheets of linked tetrahedra (T) and sheets of linked octahedra (O). The T sheet consists of Click on Term to Read More, magnetiteFe oxide, Fe2+Fe3+2O4, containing oxidized iron (Fe3+) found in the matrix of carbonaceous chondrites and as diagnostic component in CK chondrites. In CK chondrites, magnetite is typically chromian, containing several wt. % Cr2O3. Click on Term to Read More, and pyrrhotiteIron sulfide group of minerals whose composition ranges widely between its end members pyrrhotite (Fe7S8) whose crystal structure is monoclinic, and troilite (FeS) whose crystal structure is hexagonal. Its general formula is Fe1−xS (where x = 0 to 0.17). The troilite phase is found mainly in meteorites and in the Click on Term to Read More, but is notable in that it contains 10µm-sized Na,K–Cl crystals as well as zoned carbonates (Zolensky et al., 2015). These carbonates are composed of Ca-carbonate overlying Mn-rich cores, and are surrounded by thin Na–Mg-rich rims. The association of these alkalis with the CI-like clast in the Zag meteorite suggests a likely source for the Zag (and Monahans) halite (Zolensky et al., 2015). Mineralogically similar CI-like clasts have been identified in the H5–6 Tsukuba and the H4–5 Carancas meteorites, but to date no halite has been observed (Zolensky et al., 2016). It has been posited that the halide salts were not formed in situ, but rather was incorporated along with the clastic matrix under low temperature conditions from other regions on the H chondriteOrdinary chondrites with a high content of free Ni-Fe metal (15-19 vol. %) and attracted easily to a magnet. Their main minerals are olivine (Fa16-20) and the orthopyroxene bronzite (Fs14.5-18.5), earning them their older name of bronzite chondrites. Chondrules average ~0.3 mm in diameter. Comparison of the reflectance spectra of Click on Term to Read More parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More (Zolensky et al., 2000; Bridges et al., 2004).

Putative halite salts within the H3–4 matrix component (top portion) of a slice of Zag exhibiting oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More from the terrestrial environment.
Specimen acquired from R.A. Langheinrich Meteorites
An alternative scenario was proposed by Fries et al. (2013), in which the halite was derived from a nearly pure NaCl-H2O brine on a large, ancient, cryovolcanically-active body such as the dwarf planet Ceres. Analogous geysering of water with embedded halides has been observed on Saturn’s moon Enceladus (Zolensky et al., 2013). Queenie et al. (2018) propose an origin for the halite as a surface evaporite, while the primitive organic matter and the amino acids being synthesized from formaldehyde and amonia in a low-temperature aqueous environment. It is notable that the H-chondrite-like asteroid 6 Hebe has an orbitThe elliptical path of one body around another, typically the path of a small body around a much larger body. However, depending on the mass distribution of the objects, they may rotate around an empty spot in space • The Moon orbits around the Earth. • The Earth orbits around Click on Term to Read More that overlaps that of Ceres, making Ceres a reasonable source for the exogenous halite. Such an orbital resemblance would also have been important to the preservation of halite crystals during low-impact transference from Ceres to 6 Hebe (Fries et al., 2013).

The formation of halite on its original parent body might have occurred through aqueous alteration processes, initiated by aqueous fluid production through the dehydration of existing phyllosilicates in impact-heating events. Unlike the sylvite-containing halite crystals found in Monahans (1998), the high purity of the NaCl brine in Zag could indicate an origin through the evaporationProcess in which atoms or molecules in a liquid state (or solid state if the substance sublimes) gain sufficient energy to enter the gaseous state. Click on Term to Read More and concentration of asteroidal impact-accumulated ices at a depth of a few km. As the halide brine became supersaturated, precipitation of halite occurred along with trapping of fluid inclusions at temperatures of <70°C; secondary fluid inclusions were trapped along fractures (Busfield et al., 2004). Subsequent impact gardening mixed the halite, H5–6 clasts, and H3–4 matrix material, and these components were emplaced together near the surface. An alternative scenario for the production of halite was proposed by Jones et al. (2011). They suggest that increased heating at depth (i.e., involving the petrologic typeMeasure of the degree of aqueous alteration (Types 1 and 2) and thermal metamorphism (Types 3-6) experienced by a chondritic meteorite. Type 3 chondrites are further subdivided into 3.0 through 3.9 subtypes. 6 horizon) caused degassing and the production of a halogen-rich, water-poor fluid. Next, this fluid reacted with merrillite to form F,Cl-bearing apatite, which ultimately led to the consolidation of F in apatite and the enrichment of Cl in the fluid. As the Cl-rich fluid ascended into the horizon consisting of petrologic type 4 material, it infiltrated merrillite, forming Cl-rich apatite which was subsequently enriched in Na. Thereafter, the fluid precipitated halite at the H4 horizon. The halite was formed after the silicates underwent thermal metamorphism but before brecciationThe formation of a breccia through a process by which rock fragments of of various types are recemented or fused together. Click on Term to Read More of the matrix in the outer regolith 4.25 b.y. ago. The halite crystals in Zag (see photo below) attained their blue-to-purple coloration through cosmic-ray-induced electron trapping in Cl ions. Recent efforts to date these crystals in Zag have utilized radioisotope chronometry employing 129Xe data obtained from the halite. This isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More is produced by the decay of 129I (half-lifePeriod of time required for 50% (½) of the atoms of a radioactive nuclide in a sample to decay. After two half-lives, 25% ( ½ x ½ = 1/4) of the original radioactive nuclide will remain. After three half-lives, 12.5% ( ½ x ½ x ½ = 1/8) of the original radioactive nuclide will remain. Click on Term to Read More = 16 m.y.) which was only present in the early Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids.. From the fixed rate of decay of 129I into 129Xe, and the proportions of each isotope present in the halite, the age of the halite was calculated relative to other isotopic dating systems. An ancient age in the range of 4.561–4.559 b.y. was found, providing evidence that water was available on some asteroids only ~2 m.y. after the birth of the Solar SystemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with. Radioisotope studies also indicate that the I–Xe system was reset ~4.546 b.y. ago, likely by shock or aqueous alteration processes in an impact event that followed the deposition of halite (Ebisawa and Nagao, 2005). Zag consists of four lithologies, representing types H4 through H6, that are present in the following approximate modal abundances: 65% light-colored, chondrule-bearing, angular clasts (S2–4); 25% gray-colored, chondrule-bearing, clastic matrix (S2–3); 10% dark-colored, chondrule-bearing, angular clasts (S4); and <1% impact-melt-rock clasts. If any original H3 material is still present, it is rare. Most lithologies exhibit various degrees of silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the darkening, produced from curvilinear blebs and veinlets of impact-mobilized FeNi-metal, troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More, and chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More within and around the silicates. The dark-blue halite crystals have been found only within the clastic matrix, which is also the site of solar noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More concentrations. Many Zag fragments display slickensides, produced by the shearing motion of adjacent faultFracture along which there has been movement or displacement. Click on Term to Read More faces. This shearing motion is lubricated through the accumulation of very fine particles, resulting in a smooth polished surface. Consistent with the fact that Zag is a recent witnessed fall, it has a weathering grade of W0/1. The S(IV)-type asteroid 6 Hebe is thought to be the probable parent body of the H-type ordinary chondrites, and possibly of the IIE iron meteorites as well. Hebe is a 116-mile-diameter asteroid located next to both the 3:1 and ν6 resonances, providing an efficient and rapid transfer mechanism into Earth-crossing orbit and a significant source of meteorites to Earth. Based on spectrographic and mineralogical data for more than 1,000 near-Earth asteroids, Binzel et al. (2016) determined the probable main beltBelt located between 2.12 and 3.3 AU from the Sun and located between the orbits of Mars and Jupiter containing the vast majority of asteroids. The asteroid belt is also termed the main asteroid belt or main belt to distinguish it from other asteroid populations in the Solar System such Click on Term to Read More source region for each of the ordinary chondriteWork in Progress Ordinary chondrites (OCs) are the largest meteorite clan, comprising approximately 87% of the global collection and 78% of all falls (Meteoritical Society database 2018)1. Meteorites & the Early Solar System: page 581 section 6.1 OC of type 5 or 6 with an apparent shock stage of S1, Click on Term to Read More groups (see diagram below).
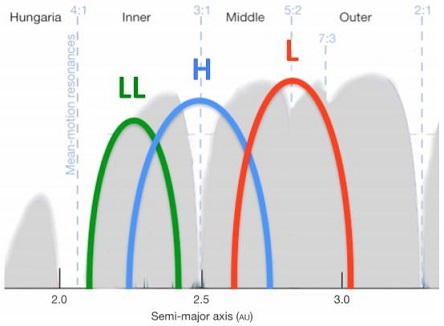
Diagram credit: Binzel et al., 47th LPSC, #1352 (2016) The average CRE age of Zag based on 3He, 21Ne, and 38Ar is calculated to be 5.1 (±0.5) m.y., close to the peak of the latest of the three breakup events determined for H chondrites (Eugster et al., 2007). It has been estimated that 6 Hebe could contribute ~10% of the meteorite flux to Earth and that it may be the source of one of the major ordinary chondrite groups. Models show that by mixing a component of 40% FeNi-metal with 60% H5 chondrite, an exact match to the spectra of 6 Hebe is produced. The IIE irons could then be created through impact melting on the metal-rich H chondrite parent body to produce melt sheets or pods near the surface. Read more about the formation of IIE irons on the Miles page. Be that as it may, hydrocode models reveal inconsistencies between expected and observed CRE ages based on the scenario of direct injection into resonances. The steady delivery of H chondrite material from 6 Hebe to Earth also remains unexplained. Current studies by Rubin and Bottke (2009) have led to the conclusion that family-forming events resulting in large meteoroidSmall rocky or metallic object in orbit around the Sun (or another star). reservoirs having homogenous compositions, and which are located near dynamical resonances such as the Jupiter 3:1 mean motion resonance, are a more likely source of the most prevalent falls including the H chondrites. See further details on the NWA 2898 page. The specimen of Zag shown above is a 1.0 g fragment displaying signs of brecciation.

A section of Zag displaying blue halite crystals.
Photo by Walt Radomsky. Courtesy of R. A. Langheinrich Meteorites







