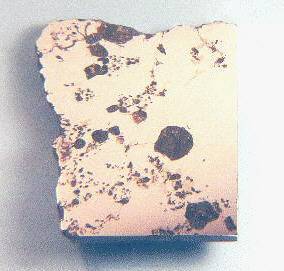
Vermillion
PyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More PallasiteOne of two main classes of stony-iron meteorite, the other being mesosiderites. Pallasites are igneous in nature and characterized by crystals of olivine, sometimes peridot (green gem quality clear olivine crystals), embedded in a matrix of Fe-Ni metal. The type specimen, weighing 680 kg, was found in the mountains near Click on Term to Read More
Vermillion grouplet
Found May, 1991, recognized 1995
39° 44.18′ N., 96° 21.68′ W. A 34.36 kg mass was found by M. and G. Farrell while planting in a grain field in Marshall County, Kansas, in the vicinity of the Black Vermillion River. This was purchased by a dealer with the belief that it was a Brenham mass. Upon cutting, it was discovered to be unique from Brenham. Vermillion consists of ~86 vol% FeNi-metal and ~14 vol% silicates with grains much smaller than normal (Boesenberg et al., 1995). The silicates consist of olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More (~93 vol%), orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More (~5 vol%), chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More (~1.5 vol%), and merrillite (~0.5 vol%). Vermillion was compared to the pallasite Yamato 8451 (54.9 g) which has a very similar silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the composition consisting of olivine (~94 vol%), orthopyroxene (~4.8 vol%), clinopyroxene (~1.1 vol%), and merrillite (~0.1 vol%) reported by Yanai and Kojima (1995). Pyroxene accounts for ~0.7 and ~1.6 vol% of the silicate fraction of Vermillion and Y-8451 respectively, the remainder being mostly olivine. By comparison, the main-group pallasites contain all olivine with only trace amounts of pyroxene. The coexistence of both olivine and pyroxene in these two pallasites might indicate a lower crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More temperature. In light of the compositional and isotopic similarities between Vermillion and Y-8451, Boesenberg et al. (1995) proposed they be recognized as a grouplet.
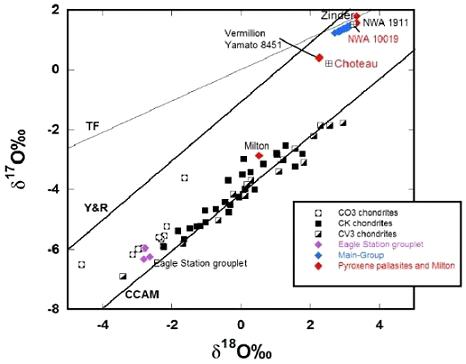
Diagram credit: Gregory et al., 47th LPSC, #2393 (2016) However, significant differences that exist between these three Vermillion pallasites are not yet resolved, including differences in texture (Y-8451 contains 4× the vol% of silicates as Vermillion) and siderophile trace elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More composition, as well as the presence of the carbide coheniteFe-Ni-Co carbide, (Fe,Ni,Co)3C, that occurs as an accessory constituent in several iron meteorites, and coarse octahedrites with < 7 wt. % Ni. Click on Term to Read More in Vermillion. In addition, although Vermillion, Y-8451, and Choteau all contain both low- and high-Ca pyroxenes of similar compositions, some differences are evident. Vermillion and Y-8451 contain both pyroxene types in the form of large grains, inclusions in olivine, and grains bordering olivine. However, in Choteau high-Ca pyroxene has only been identified as inclusions in olivine, while low-Ca pyroxene is present as both individual grains and along boundaries of high-Ca pyroxene grains (Gregory et al., 2016). Furthermore, plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More has not been found in either Vermillion or Y-8451, but is present in Choteau as inclusions in both low- and high-Ca pyroxene and as small veins in low-Ca pyroxene. Notably, plagioclase is also present in the ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More pyroxene pallasite NWA 10019, but it is compositionally distinct to that in Choteau. The olivine fayalitePure* iron end-member (Fe2SiO4) of the olivine solid solution series and an important mineral in meteorites. When iron (Fe) is completely substituted by magnesium, it yields the the pure Mg-olivine end-member, forsterite (Mg2SiO4). The various Fe and Mg substitutions between these two end-members are described based on their forsteritic (Fo) Click on Term to Read More composition of these pyroxene-bearing pallasites plots at the magnesian end of the main-group pallasite range. As a comparison, the Eagle Station pallasite group has the most ferroan composition, as well as a high Ge/Ga ratio in the metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More and a unique O-isotope composition. The high Ir content in the Eagle Station pallasites suggests crystallization from the inner coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More region of its parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More, below the core–mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More interface in which the main-group and pyroxene-bearing meteorites probably formed on their respective parent bodies. Siderophile trace element and oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More isotopic compositions clearly resolve the pyroxene-bearing meteorites from the main-group and Eagle Station pallasites, and therefore they represent additional parent bodies on which pallasite-like textures were formed. In addition to the Vermillion trio, several other pyroxene-bearing meteorites have been found and studied. A 46 g pyroxene-rich pallasite named Zinder was found in Niger in 1999. It contains 28 vol% pyroxene and 27 vol% olivine in a network of FeNi-metal. In 2003, a 53 g pyroxene-rich pallasite designated NWA 1911 was purchased in northwest Africa. It has a modal composition of about 24% FeNi-metal and 75% silicates, with the silicates consisting of 34.5% orthopyroxene and 40.2% olivine. Separate fragments of the ungrouped pyroxene-bearing pallasite NWA 10019, weighing together 606 g, were found in 2015. On a Ni vs. AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More coupled diagram, Vermillion plots just outside of the low-Au end of the IAB main group irons, and also plots along an extention of the low-Au, medium-Ni (sLM) subgroup into lower Au compositions (Wasson and Kallemeyn, 2002). Furthermore, the O-isotopic composition of Vermillion is within the range of IAB irons, and therefore, Vermillion could be considered genetically related to members of the low-Au division of the IAB iron-meteorite complex. Based on all of the data gathered so far, it could be concluded that the pallasites in our collections represent at least seven separate parent bodies: 1) main-group; 2) Eagle Station group; 3) Milton; 4) Choteau + Vermillion + Y-8451; 5) Zinder + NWA 1911; 6) NWA 10019; 7) LoV 263. In addition, several pallasites with anomalous silicates (e.g., Springwater) and anomalous metal (e.g., Glorieta Mountain) could possibly increase the number of unique parent bodies. A beautiful etched slice of Choteau is shown in ‘MeteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More Picture of the Day’ for June 20, 2013, courtesy of Ruben Garcia. The specimen of Vermillion pictured above is a 67.9 g partial slice. The photo below shows a close-up of the etched reverse side.