Iron, IAB complex, Pitts grouplet

Found Spring 1953
42° 20′ 48′ N., 90° 10′ 6′ W. A mass of 48.2 kg was plowed up on Henry Albrecht’s farm ~2 km west of Woodbine, Illinois. The weathered angular mass measuring 30 × 27 × 17 cm was without its fusion crustMelted exterior of a meteorite that forms when it passes through Earth’s atmosphere. Friction with the air will raise a meteorite’s surface temperature upwards of 4800 K (8180 °F) and will melt (ablate) the surface minerals and flow backwards over the surface as shown in the Lafayette meteorite photograph below. Click on Term to Read More and heat-affected zone, indicating a very long terrestrial residence.
The IAB iron-meteorite complex, recently proposed by Wasson and Kallemeyn (2002), comprises iron meteorites from the former IAB-IIICD group, as well as numerous related irons. Many of the members contain
silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the inclusions with chondritic compositions. Woodbine is a low-Au member of the IAB complex that is closely related to the main group. On a Ni–
AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More diagram, a grouplet of three members—Woodbine, Colfax, and Pitts—has been resolved in an area intermediate between the sLL and sLM subgroups, but with higher Ni contents, and they were designated the Pitts grouplet. A new study conducted by Worsham
et al. (2016) coupling Pd
vs. other HSEs found that the Pitts grouplet and the sLM subgroup both show enrichments in Pd abundances, but otherwise they are considered to have experienced distinct petrogenetic histories.
The
metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More in Woodbine has a small-grained polycrystalline texture, exhibiting independently oriented, fine-textured Thomson (Widmanstätten) structures. Bulk analysis reveals that 20.4 wt% of Woodbine consists of non-metal phases composed of 7.6%
enstatiteA mineral that is composed of Mg-rich pyroxene, MgSiO3. It is the magnesium endmember of the pyroxene silicate mineral series - enstatite (MgSiO3) to ferrosilite (FeSiO3). Click on Term to Read More, 4.2% Mg-rich
olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More, 2.6% Na-rich
anorthiteRare compositional variety of plagioclase and the calcium end-member of the plagioclase feldspar mineral series with the formula CaAl2Si2O8. Anorthite is found in mafic igneous rocks such as anorthosite. Anorthite is abundant on the Moon and in lunar meteorites. However, anorthite is very rare on Earth since it weathers rapidly Click on Term to Read More, and 1.1% diopside which form clusters up to 6 mm wide.
SchreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More forms rims on silicate grains, and
troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More has been melted by shock and injected into the cracks of the silicates, cementing individual grains into large aggregates. Minor
graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More occurs between troilite and
kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More and as rims around silicate grains.
CarbonElement commonly found in meteorites, it occurs in several structural forms (polymorphs). All polymorphs are shown to the left with * indicating that it been found in meteorites and impact structures: a. diamond*; b. graphite*; c. lonsdalite*; d. buckminsterfullerene* (C60); e. C540; f. C70; g. amorphous carbon; h. carbon nanotube*. Click on Term to Read More is also present as
coheniteFe-Ni-Co carbide, (Fe,Ni,Co)3C, that occurs as an accessory constituent in several iron meteorites, and coarse octahedrites with < 7 wt. % Ni. Click on Term to Read More and
haxoniteIron-nickel carbide, (Fe,Ni)23C6, found in iron meteorites. Click on Term to Read More.
Dey
et al. (2019) employed
17O and ε
54Cr values for several irons and their associated silicates/oxides to investigate i) if each iron and its associated phases originated on a common
parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More (
i.e., an endogenous mixture of
coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More and
mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More vs. an exogenous mixture through impact), and ii) if any genetic connection exists between the irons and other
meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More groups (
e.g., IAB with winonaites, IIE with H
chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, and Eagle Station pallasites with CK chondrites). Three IAB irons were employed in the study, and it was demonstrated on a coupled diagram that although the ε
54Cr values for the iron component plot in the
winonaitea partially differentiated asteroid that was disrupted just as it began to form an Fe core and a silicate-rich crust. This disrupting impact mixed silicates into molten Ni-Fe metal forming the silicated IAB irons, and mixed olivine-rich residues of partial melts into unmelted silicates, forming the winonaites. A few winonaites Click on Term to Read More field, values for the silicate component plot in a distinct region on an O–Cr coupled diagram (see diagram below). From these results they ascertained that the the IAB silicated irons formed through an impact-generated mixture comprising iron from a winonaite-related parent body and silicate from an unrelated and otherwise unsampled parent body. Incorporation of the silicates into the FeNi-metal host took place at a depth greater than 2 km, allowing time for a Thomson (Widmanstätten) structure to develop during a long cooling phase.
Fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More occurred in some large molten metal pools, followed by very slow cooling, to produce the broad range of features found in certain IAB meteorites (
e.g., silicate-poor, graphite–troilite-rich inclusions and extremely high Ni contents). Other results from their study can be found on the
Miles and
Eagle Station pages.
17O
vs. ε
54Cr for Irons and Pallasites
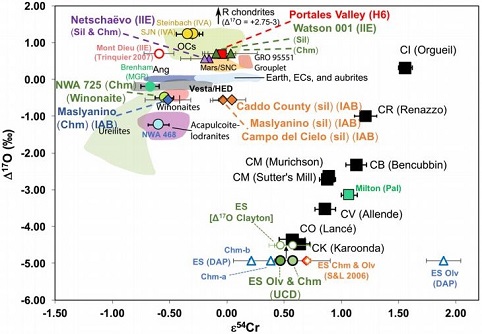 click on photo for a magnified view
click on photo for a magnified view
Diagrams credit: Dey
et al., 50th LPSC,
#2977 (2019)
The composition of Woodbine is consistent with mixing, compression, and annealing of incompletely segregated metal and silicate on a small chondritic asteroid which never experienced temperatures high enough for complete melting to occur. Upon cooling, the graphite and schreibersite precipitated on the silicate grains, followed by kamacite nucleation, which gradually developed into a Thomson (Widmanstätten) structure. Neumann bands were formed by the impact shock that dislodged the mass from the asteroid. The absolute I–Xe retention age for Woodbine, relative to the Shallowater standard, was calculated to be 4.5659 (±0.0003) b.y (Niemeyer, 1979).
In a study of
iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More exposure histories, Welten
et al. (2008) found that Pitts exhibits a complex exposure history comprising two stages. During the first stage of irradiation, which involved high shielding at a depth of 63–77 cm within an object >2 m in diameter, cosmogenic
noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More data indicate a CRE age of 600 (+190/–150) m.y. A second stage irradiation lasting only ~0.7 (±0.2) m.y. occurred on a <40 cm diameter body. The investigators argue that the cosmogenic
radionuclideRadioactive isotope - Atomic nuclide that decays radioactively . Click on Term to Read More and noble gas data for Pitts are consistent with a scenario in which a fragment was ejected during a minor impact on a km-sized near-Earth object (NEO), followed by its rapid delivery to Earth. A possible source object for the Pitts meteorite is the Earth-crossing M-type asteroid 1986 DA, itself possibly derived from a larger
main beltBelt located between 2.12 and 3.3 AU from the Sun and located between the orbits of Mars and Jupiter containing the vast majority of asteroids. The asteroid belt is also termed the main asteroid belt or main belt to distinguish it from other asteroid populations in the Solar System such Click on Term to Read More object. Since 1986 DA has a semi-major axis that is close to the 5:2 mean motion resonance with Jupiter, one of the M-type asteroids similarly located such as 16 Psyche and 216 Kleopatra might be the source parent body of the Pitts iron, and by association, the Woodbine and Colfax irons.
Woodbine is a fine
octahedriteMost Common type of iron meteorite, composed mainly of taenite and kamacite and named for the octahedral (eight-sided) shape of the kamacite crystals. When sliced, polished and etched with an acid such as nitric acid, they display a characteristic Widmanstätten pattern. Spaces between larger kamacite and taenite plates are often Click on Term to Read More of the IAB iron-meteorite complex. To learn more about the relationship within the IAB complex and among other iron chemical groups, see the
Appendix Part III. The specimen of Woodbine pictured above is a 9.7 g partial slice containing coarse silcate inclusions.








