Iron, IIIA, octahedriteMost Common type of iron meteorite, composed mainly of taenite and kamacite and named for the octahedral (eight-sided) shape of the kamacite crystals. When sliced, polished and etched with an acid such as nitric acid, they display a characteristic Widmanstätten pattern. Spaces between larger kamacite and taenite plates are often Click on Term to Read More
Found 1818
76° 8′ N., 64° 56′ W. At least 8 masses of this meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More have been recovered from the glacial region of Greenland having a total weight of 58 tons, the largest combined mass of any other recovered meteorite. The most recent found mass was a 20-ton mass named Agpalilik found in 1963. It has undergone 0.5 mm of corrosion on the above ground portion with more than 2 mm of the surface lost on portions below the soil line. Chlorides are a significant invasive elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More contributing to the deterioration.
The
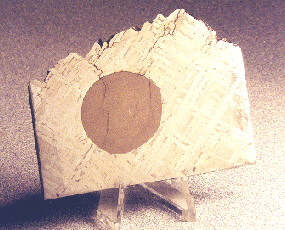
troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More inclusions, a cross-section of which is pictured above, form parallel, sausage-shaped formations within the meteorite and are derived from a trapped melt component. These troilite inclusions represent 5.6 vol% of the total mass of Cape York. A simple
fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More model for the IIIAB group gives an estimate for the initial S-content of the molten
coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More of 12 (±1.5) wt%, and indicates that most of the core material formed from the later crystallized, S-rich residual liquid is not represented in our collections (N. Chabot, 2004). Low-density phosphates and high-density
metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More grains are present in the troilite nodules opposite each other, reflecting the direction of the gravitational field on the
parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More. The nitride carlsbergite [CrN] was first discovered in the Cape York meteorite. Notably, an undifferentiated
silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the inclusionFragment of foreign (xeno-) material enclosed within the primary matrix of a rock or meteorite. Click on Term to Read More, similar to the type that occur in IAB complex irons, was discovered in the IIIAB Puente del Zacate iron; it is unclear how this occurred (Ruzicka, 2014).
The IIIAB iron group constitutes ~33% of all iron meteorites recovered. Structural evidence shows that the meteorite went through a molten stage on its parent body. Based on cooling rate models, the diameter of the parent body is estimated to have been ~100 km, taking ~50 m.y. for the core to solidify. In a study by Yang
et al. (2006, 2010), a wide range of cooling rates was found for IIIAB irons, from 60° to 340K/m.y. They propose that the IIIAB parent body had its silicate
mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More mostly stripped off during one or more impact events prior to
kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More formation and while it was still molten, and that it crystallized from the surface towards the core resulting in lower cooling rates for the more highly insulated, high-Ni subset. The Hf–W chronometer indicates that the core formed ~1.2 m.y. after
CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More, while the Pd–Ag chronometer indicates that cooling below the closure temperature for this isotopic
systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with occurred by ~4 m.y. after CAIs (Mathes
et al., (2015). Therefore, they reasoned that given a large parent body size of ~100 km, the removal of the mantle by impacts would have necessarily occurred prior to ~4 m.y. after CAIs. Notably, a genetic relationship between groups IIIAB and IIIE has been posited based primarily on matching Cu isotopic systematics (Bishop
et al., 2012).
The O-isotopic composition of chromites was ascertained for Cape York and other IIIAB irons (Franchi
et al., 2013). Curiously, the values show that Cape York (–0.27‰) has a discrepant O-isotopic composition from the others studied (–0.18‰), while the main group pallasites have an identical O-isotopic composition to the others (–0.18 (±0.02) ‰). The different value for Cape York was ascribed by the investigative team to either multiple sources for the chromites, structural diversity of the IIIAB parent body, or an origin for Cape York on a separate parent body.
Trace-element studies along with metal and O-isotopic compositions of the main-group pallasites are consistent with those of the late-crystallized (high-Au, high Ni, reflecting ~80% core
crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More) residual melts of the IIIAB iron core. The enrichment of these pallasites in the
volatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). and refractory siderophiles Ga, Ge, and Ir relative to the IIIAB group could have occurred during the crystallization of a sulfide-rich liquid fraction at the core–mantle boundary. Still, it is now considered more likely that this enrichment observed in pallasites occurred during the condensation of a metallic melt gas phase concentrated within voids which formed by core contraction and mantle collapse during cooling; moreover, recent studies rule out a core–mantle boundary formation scenario for pallasites (Yang and Goldstein, 2006, 2010). Based on the size of the island phase in the cloudy zone of these pallasites, the metallographic cooling rates appear to have been significantly lower than those of IIIAB irons. In their measurement of high-Ni particles within the cloudy zone of several main-group pallasites and IIIAB irons, Yang
et al. (2007) found that a correlation with Ni exists only in the IIIAB irons. Based on the significantly larger high-Ni particle size in the pallasites (105–188 nm)
vs. the IIIAB irons (47–71 nm), they determined that the cooling rate was ~2.5–25× lower for the pallasites, with the wide range suggesting that a large thermal heterogeneity existed within the
pallasiteOne of two main classes of stony-iron meteorite, the other being mesosiderites. Pallasites are igneous in nature and characterized by crystals of olivine, sometimes peridot (green gem quality clear olivine crystals), embedded in a matrix of Fe-Ni metal. The type specimen, weighing 680 kg, was found in the mountains near Click on Term to Read More zone. In addition, the Re–Os chronometer suggests that pallasites formed 60 m.y. later than IIIAB irons, raising further doubts about a IIIAB core–mantle origin for main-group pallasites (E. Scott, 2007). Further information on the formation of the main-group pallasites can be found on the
Imilac page.
Studies of the Pd–Ag systematics of the Cape York IIIA iron and the Grant IIIB iron by Matthes
et al. (2013, 2014) indicate that the IIIAB core cooled rapidly, within the first ~2 m.y. of
Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. history using IVA Muonionalusta as the anchor. It is considered most likely that the core crystallized inwards, with crystallization of Cape York succeeding that of Henbury and preceeding that of Grant (Matthes
et al., 2014). It was shown that each of these meteorites cooled below the Pd–Ag closure age within ~1 m.y. of each other. The corrected I–Xe isotopic systematics for Cape York yields a CRE age of 82 (±7) m.y. (Marti
et al., 2004; Mathew and Marti, 2009).
To learn more about the relationship between this and other iron chemical groups, click
here. The photo above shows a 57.2 g Cape York specimen. The 20-ton Agpalilik mass is pictured below.

Photo courtesy University of Copenhagen—Geological Museum








