ALH 84001
OrthopyroxeniteA rock composed primarily of orthopyroxene. Non-terrestrial orthopyoxenites include diogenites and a single martian meteorite, ALH 84001, that was found in the Allan Hills region of Antarctica in 1984. ALH 84001 is a cumulate rock consisting of 97% coarse-grained, Mg-rich orthopyroxene, with small amounts of plagioclase, chromite, and carbonate. It Click on Term to Read More
(with geochemical affinities to shergottitesIgneous stony meteorite with a Martian origin consisting mainly of plagioclase (or a shocked glass of plagioclase composition) and pyroxene. They are the most abundant type of SNC meteorites and the type member is the Shergotty meteorite, which fell in India in 1865. Shergottites are igneous rocks of volcanic or Click on Term to Read More)
NASA photo #S85-39570 Found December 27, 1984
76° 43′ S., 159° 40′ E. Allan Hills 84001 was found during the 1984 annual field season of the Antarctic Search for Meteorites (ANSMET) by Robbie Score during a search by snowmobile in the Allan Hills region of the Far Western Icefield, Antarctica. The 1,931 g stone was originally classified as a diogeniteDiogenites belong to the evolved achondrite HED group that also includes howardites and eucrites. They are named after the Greek philosopher Diogenes of Apollonia, of the 5th century BCE, who was the first to suggest that meteorites come from outer space (a realization forgotten for over 2,000 years). They are Click on Term to Read More with a weathering category of A/B (minor to moderate rustiness), and a fracturing category of B (moderate cracks). After a more thorough analysis, it was found that the meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More was unusual in its Fe/Mn ratio, its Fe+3 content in chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More, the presence of pyrite instead of troiliteBrass colored non-magnetic mineral of iron sulfide, FeS, found in a variety of meteorites. Click on Term to Read More, and an oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More analysis unlike that of diogenitesDiogenites belong to the evolved achondrite HED group that also includes howardites and eucrites. They are named after the Greek philosopher Diogenes of Apollonia, of the 5th century BCE, who was the first to suggest that meteorites come from outer space (a realization forgotten for over 2,000 years). They are Click on Term to Read More, and it is now recognized as having a martian origin. Although ALH 84001 is an orthopyroxenite, and as such was characterized by the Planetary Chemistry Laboratory at Washington University as a subgroup of the nakhlites, its parental source magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More has a composition that is consistent with the same mixtures of depleted and enriched REEOften abbreviated as “REE”, these 16 elements include (preceded by their atomic numbers): 21 scandium (Sc), 39 Yttrium (Y) and the 14 elements that comprise the lanthanides excluding 61 Promethium, an extremely rare and radioactive element. These elements show closely related geochemical behaviors associated with their filled 4f atomic orbital. Click on Term to Read More end-member components that are used in a geochemical classification of the shergottites (Lapen et al., 2012). It was determined that the source magma of ALH 84001 contained a higher proportion of the enriched REE component than all other shergottites studied thus far. Therefore, ALH 84001 might be most appropriately classified as a subgroup of the shergottites.
Based on Sm–Nd and Rb–Sr data, ALH 84001 appears to have the oldest crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More age of all martian meteorites at 4.470 (+0.035/–0.026) b.y. (Nyquist and Shih, 2013). However, calculations made by others utilizing the U–Pb and Pb–Pb isotopic systems defined a younger combined mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More age of 4.117 (±0.016) b.y., consistent also with the maskelyniteNatural glass composed of isotropic plagioclase produced during shock metamorphism (not melting) at pressures of ~30 GPa. Maskelynite is commonly found in shergottites though also found in some ordinary chondrites, HED and lunar meteorites. It is also found in association with meteorite impact craters and crater ejecta. Named after British Click on Term to Read More Ar–Ar age (4.163 [±0.035] b.y.) and the Lu–Hf age; this is probably also the time of acquisition of the natural remanent magnetization component that is observed in the meteorite. A Lu–Hf age determination by Righter et al. (2009) resulted in a young crystallization age of 4.086 (±0.030) b.y., and an age derived by Lapen et al. (2010) reflects an age of 4.091 (±0.030) b.y. Reportedly, the Lu–Hf systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with is less subject to alteration by water, shock, and heat than the Sm–Nd system, and is thought to accurately reflect the crystallization of cumulateIgneous rock composed of crystals that have grown and accumulated (often by gravitational settling) in a cooling magma chamber. Click on Term to Read More orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More derived from an LREE-enriched, late-stage parental source magma. This younger crystallization age may be associated with the Late Heavy BombardmentPeriod between ~4.0 to 3.8 Ga ago when the Moon and other objects in the Solar System were pounded heavily by wayward asteroids. The evidence for the Late Heavy Bombardment (LHB) includes the lunar maria basins and similar structures elsewhere, such as the Caloris Basin on Mercury and the great Click on Term to Read More period of the Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids.. Contrariwise, Nyquist and Shih (2013) consider the Sm–Nd radiometric system to be the more robust of the two, and they argue that it was the Lu–Hf age that reflects a resetting event.
Various radiometric dating systems indicate a younger carbonateMineral or compound containing carbon and oxygen (i.e. calcium carbonate, CaCO3, calcite). Click on Term to Read More formation age of 3.9–4.0 b.y., while a Th–Pb isochron at 2.9 b.y. attests to the late addition of Th to the ALH 84001 lithology, likely through metasomatic processes by a phosphate-rich liquid (Jagoutz et al., 2009; Albarède et al., 2009). The 4.12 b.y. crystallization age is consistent with that of a large number of enriched shergottites, including Zagami, Shergotty, RBT 04262, and NWA 1068, thought to be derived from a late-stage incompatible-element-rich residual liquid. Notably, strong similarities exist for the high Hf/Sm and Zr/Sm ratios and the trace elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More patterns among these enriched shergottites and ALH 84001 (Barrat and Bollinger, 2010). Furthermore, the similar values for ε142Nd and ε182W, and the Lu/Hf and U/Pb ratios, reflect a close affinity between shergottites and ALH 84001 and raise the possibility of a shergottiteIgneous stony meteorite with a Martian origin consisting mainly of plagioclase (or a shocked glass of plagioclase composition) and pyroxene. They are the most abundant type of SNC meteorites and the type member is the Shergotty meteorite, which fell in India in 1865. Shergottites are igneous rocks of volcanic or Click on Term to Read More precursor. Another isochron observed at 4.3 b.y. is the same as another grouping of shergottites, including QUE 94201 and NWA 1195.
ALH 84001 consists of 97% cumulate, coarse-grained, magnesian orthopyroxene. The rock is thought to have crystallized at low pressure (<0.5 GPa) at a depth of several tens of km under slightly reducingOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More conditions (QFM –2.7), and under the influence of carbonElement commonly found in meteorites, it occurs in several structural forms (polymorphs). All polymorphs are shown to the left with * indicating that it been found in meteorites and impact structures: a. diamond*; b. graphite*; c. lonsdalite*; d. buckminsterfullerene* (C60); e. C540; f. C70; g. amorphous carbon; h. carbon nanotube*. Click on Term to Read More buffering (Righter et al., 2008). It is considered likely to be a sample from the 4+ b.y. old southern hemisphere of Mars, rather than the youger, lava-covered, northern hemisphere. Alternatively, a rayed craterBowl-like depression ("crater" means "cup" in Latin) on the surface of a planet, moon, or asteroid. Craters range in size from a few centimeters to over 1,000 km across, and are mostly caused by impact or by volcanic activity, though some are due to cryovolcanism. Click on Term to Read More 6.9 km in diameter named Gratteri, located near Memnonia Fossae southwest of the Tharsis volcano, could be the source of ALH 84001 (Tornabene et al., 2006). This crater, exhibiting more than 30 rays having lengths up to 595 km, was formed by an oblique impact ~20 m.y. ago. The impact excavated Noachian-aged rock (a period extending from the birth of Mars to ~3.5 b.y. ago) is consistent with the ancient age of ALH 84001. Based on data from the Infrared Mineralogical Mapping Spectrometer aboard the Mars Express orbiter, orthopyroxene-rich terrain of appropriate Noachian age has only been identified in regions of Syrtis Major and in the northwest region of Hellas basin (Ody et al., 2013).
Following the formation of carbonate and maskelynite, a rapid, localized, high-temperature (>1400°C) shock event occurred 1.158 (±0.110) b.y. ago (Cassata et al., 2010). From models of the time period following this localized event, temperatures for the ALH 84001 rock were maintained at either ~80°C for 10 m.y. if residing under an ejecta blanketGenerally symmetrical apron of ejecta surrounding a crater; it is thick at the crater's rim and thin to discontinuous at the blanket's outer edge. Click on Term to Read More, or ~330°C for several days if residing near the surface (Cassata et al., 2010). Remagnetization occurred during this shock heating event, causing the heterogeneous pattern of magnetization observed. Following the ejection of the rock from Mars ~12 m.y. ago, temperatures were calculated to be a maximum ~75°C or ~320°C, corresponding to a duration of 10 m.y. and several days, respectively.
A shock metamorphicRocks that have recrystallized in a solid state due to changes in temperature, pressure, and chemical environment. Click on Term to Read More event is recorded in the U–Th–Pb age of the phosphates, in the Ar–Ar shock age from maskelynite, and in other isochrons, each indicating an age of ~4.0 m.y (Terada et al., 2003). Treiman (1998) determined that ALH 84001 experienced 4–5 separate impact events. During the third impact event, which succeeded carbonate formation, the rock experienced its greatest heating, increasing to a temperature of at least 529°C, and perhaps as high as 800–900°C; the latter high temperature is consistent with the feldspathic melts present in the rock (Domeneghetti et al., 2007). The maskelynite has a refractive index that corresponds to a shock pressure of 32 (±1) GPa (Fritz et al., 2005). It may be presumed that during this significant impact event, the rock was launched into a suborbital trajectory and then covered by an ejectaFractured and/or molten rocky debris thrown out of a crater during a meteorite impact event, or, alternatively, material, including ash, lapilli, and bombs, erupted from a volcano. Click on Term to Read More blanket after landing. It is likely that this is the period when the Fe-rich carbonate globules were decomposed to fine-grained, whisker-shaped magnetiteFe oxide, Fe2+Fe3+2O4, containing oxidized iron (Fe3+) found in the matrix of carbonaceous chondrites and as diagnostic component in CK chondrites. In CK chondrites, magnetite is typically chromian, containing several wt. % Cr2O3. Click on Term to Read More.
The rock was then rapidly cooled over a short duration of time, probably measured in minutes for a rock situated very close to the surface, preserving the carbonate and the observed chemical zoning. Thereafter, a cooling rate of ~35°C/year was established, as indicated by the orthopyroxene geospeedometer (Domeneghetti et al., 2007). At a depth of ~6 m, cooling proceeded over the next couple of hundred years. During one or two subsequent impact events, a post-shock temperature increase of 100–110°C (to at most 350–500°C) was attained, after which the ALH 84001 lithological unit did not experience high metamorphic temperatures again, not even during its subsequent ejection from the planetThe term "planet" originally comes from the Greek word for "wanderer" since these objects were seen to move in the sky independently from the background of fixed stars that moved together through the seasons. The IAU last defined the term planet in 2006, however the new definition has remained controversial. Click on Term to Read More.
Recently, thermal emission spectrometry performed by the Mars Global Surveyor has located a region in Eos ChasmaTerm applied to a deep, elongated, steep-sided depression (canyon) on a planetary surface (e.g., Candor Chasma on Mars). Click on Term to Read More that contains orthopyroxene compositionally similar to that in ALH 84001, hinting at a possible point of origin. Within the orthopyroxene, mm- to cm-wide crushed and annealed fracture zones are present, indicating that this meteorite was subjected to an intense, localized shock event of short duration after it was cooled in an igneous plutonicGeology: Igneous intrusive body that forms when magma is injected into host rocks and solidifies. Plutons occur in the crust of asteroids undergoing differentiation or planets. Named after Pluto, the Roman god of the underworld. Plutonic rocks are the rocks found within a pluton. Astronomy: Category of planet including all Click on Term to Read More environment. No evidence of subsequent metamorphism was obseved. The fracture zones contain µm- to sub-µm-sized angular, rounded, and euhedral chromite grains; some appear to be stringers of shock-dispersed larger grains, while others likely exsolved from a Cr-rich melt phase. In addition, orange-colored, rosette-, slab-, or disk-shaped carbonate inclusions are present, some of which have rims consisting of two Fe-rich black zones sandwiching a white Mg-rich zone.
These chromites and carbonates are thought to have precipitated ~4 b.y. ago from a heated, shock mobilized solution, consistent with the observed chemical heterogeneities and microstructures (Barber and Scott, 2006). However, other investigators believe the larger carbonates precipitated from a low-temperature, saturated solution. In support of a low temperature environment for carbonate formation, Theis et al. (2008) constrained the temperature of carbonate precipitation to 83°C (±67°C), based on the degree of Fe isotope fractionationConcentration or separation of one mineral, element, or isotope from an initially homogeneous system. Fractionation can occur as a mass-dependent or mass-independent process. Click on Term to Read More affecting the zoned carbonates relative to the Fe isotopic composition of martian silicates with which they were initially in equilibriumTerm used to describe physical or chemical stasis. Physical equilibrium may be divided into two types: static and dynamic. Static equilibrium occurs when the components of forces and torques acting in one direction are balanced by components of forces and torques acting in the opposite direction. A system in static Click on Term to Read More. Nucleation or redistribution of these phases occurred within these impact-generated fracture zones, with the slab-shaped carbonates relegated to formation within the larger fractures. Various minerals were subsequently deposited upon these carbonates, which are designated ‘magnesite–siderite–magnesite’ layers and ‘post-slab magnesites’, while chromium oxide (eskolaite) was deposited on rare silicaSilicon dioxide, SiO2. glass. The final phase in the post-shock assimilation sequence was the mobilization of molten feldspathic glass (Corrigan, 2004) and phosphate.
Usui et al. (2016) conducted a H,C,O-isotopic study of the carbonates in ALH 84001. They obtained a more precise range for the δD value of ~500–1,000‰; this value represents the H-isotopic composition of the water reservoir during carbonate formation in the Noachian period. In addition, they ascertained that the H-isotopic composition is positively correlated with the C- and O-isotopic compositions.
Nuclear track data suggest that ALH 84001 had a pre-atmospheric radius of ~10 cm, and suffered atmospheric ablationGradual removal of the successive surface layers of a material through various processes. • The gradual removal and loss of meteoritic material by heating and vaporization as the meteoroid experiences frictional melting during its passage through the atmosphere. The resulting plasma ablates the meteor and, in cases where a meteor Click on Term to Read More of over 85%. Among martian meteorites, ALH 84001 has the oldest cosmic-ray exposure ageTime interval that a meteoroid was an independent body in space. In other words, the time between when a meteoroid was broken off its parent body and its arrival on Earth as a meteorite - also known simply as the "exposure age." It can be estimated from the observed effects Click on Term to Read More of 14.7 (±0.9) m.y. It has a terrestrial age of ~13,000 years, but spent less than 500 of those years exposed on the surface of the Antarctic icefields, consistent with its low terrestrial weathering effects.
In a paper published in Science, McKay et al. (1996) presented evidence of possible biogenic activity on early Mars that was found in this martian meteoriteOver 30 of the meteorites found on Earth almost certainly came from Mars (see http://www.imca.cc/mars/martian-meteorites.htm and http://www2.jpl.nasa.gov/snc/). All but one belongs to the group known as SNC meteorites, which includes the shergottites, nakhlites, and chassignites. SNC meteorites contain minerals that crystallized within the past 1.35 to 0.15 Ga, making them Click on Term to Read More. The evidence supporting their theory included the following:
- The igneous nature of ALH 84001, along with the fact that it was penetrated by a fluid along fractures and pores, followed by secondary mineralMineral that forms through processes such as weathering, and in the case of meteorites can also include pre-terrestrial alteration. Secondary minerals in meteorites that formed during terrestrial weathering include oxides and hydroxides formed directly from metallic Fe-Ni by oxidation, phosphates formed by the alteration of schreibersite, and sulfates formed by Click on Term to Read More formation.
- The occurrence of oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More, single-domain, magnetite, and reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More iron sulfide particles, within partially disolved carbonate, possibly resulting from anaerobic microbial organisms.
- SEM and TEM images showing nanometer structures resembling terrestrial microfossils.
- An abundance of specific polycyclic aromatic hydrocarbons (PAHs) associated with the carbonates.
They wrote that although alternative explanations exist for each of these factors taken individually, when considered together in such close association, they constitute evidence for primitive life on early Mars.
According to some, an important constraint on this theory is the extremely small size of the proposed organisms, thought to be below the size needed to contain the framework of a living organism. Recently however, nannobacteria in the 50 nm size-range, with shapes resembling those found in ALH 84001, were cultured in the lab. Results of tests for DNA and cell walls were positive. Similar microfossil-like features have also been found in the Nakhla and Shergotty meteorites.
Magnetite particles extracted from carbonate globules in ALH 84001 have been characterized as irregular, prismatic, or whisker-like. The prismatic magnetites (see image below) were found to be indistinguishable from terrestrial magnetites produced by the bacteria strain MV-1 in several properties that are not associated with inorganic magnetites. Rather, these properties, regarding attributes of size, morphology, and chemistry, are uniquely characteristic of biogenic bacteria. Therefore, this particular type of magnetite may be interpreted as having a biogenic origin. In contrast, an inorganic process involving the thermal decomposition of sideriteAn obsolete term for an iron meteorite. has been proposed as the source of the single-domain magnetites, which M. S. Bell (2017) has experimentally demonstrated under shock conditions of 49 GPa and temperatures >470°C. 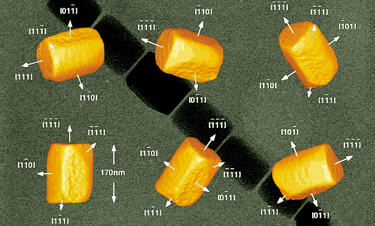
A TEM-based image showing different views of a magnetite (Fe3O4) nanocrystal from a magnetotactic bacterium. Another important constraint on this theory is the temperature of formation. McKay et al. think it was no higher than 80°C while others place it closer to 700°C. Subsequent research in this area demonstrated that high-temperature impact-produced brecciationThe formation of a breccia through a process by which rock fragments of of various types are recemented or fused together. Click on Term to Read More on ultra-mafic host rock can mobilize CO2 to form the carbonates observed. A subsequent rapid cooling of the carbonates, consistent with an impact event, would be necessary to preserve the fine-scale zoning features observed. However, the vast majority of investigations into the temperature issue concludes that the carbonates were formed at temperatures below 100°C. Of particular interest is an investigation into the natural remanent magnetismAlso called residual magnetism, refers to the permanent magnetization preserved within the ferromagnetic minerals inside rocks, like meteorites. The presence of a magnetic field assumes and requires a differentiated parent body that contained a liquid core sometime in its past. When a rock cools below its Curie temperature, it acquires Click on Term to Read More (NRM) of carbonate-containing fractures, which has found that the original magnetic field is present. Therefore, the occurrence of adjacent fragments having differently oriented magnetic fields attests to the fact that the temperature of the fractured rock was never greater than 325°C; at this point an identical magnetic orientation would form upon cooling. It can also be presumed that the carbonates formed in the fractures after the fractures were formed, and therefore, the carbonates formed at temperatures below 325°C.
Other researchers have investigated the distribution of PAHs by studying them in the martian meteorite EET 79001. This meteorite has a far younger formation age of 180 m.y., and as such, formed after the surface of Mars became devoid of water. In addition, analyses of PAHs from a carbonaceous meteorite that was recovered near ALH 84001, and of PAHs from Antarctic ice meltwater, have revealed that all of these sources of PAHs were similar. This study suggests that these sources probably reflect thousands of years of contamination from terrestrial or extraterrestrial PAHs that are present in the Antarctic ice. In support of the contamination theory, results of a time-of-flight secondary ionAtom with a net electrical charge because it has lost, or gained, one or more electrons relative to the number possessed by a neutral atom of the same element. A positively charged ion (cation) has fewer electrons than a neutral atom; a negatively charged ion (anion) has more. Click on Term to Read More mass spectrometry (TOF-SIMS) analysis revealed a correlation exists between the PAHs and terrestrial lead (Stephan et al., 2003). This study also demonstrated that there is no spatial association between the PAHs and the carbonate globules as previously claimed. Moreover, the effects of oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More from Antarctic weathering on the PAHs, resulting in a reduced alkylation, can be readily explained without reference to possible fossil martian life. Studies of other biomarkers of biologic activity including carbon, oxygen, and sulfur isotopes, suggest that they reflect cycling through the martian atmosphere or terrestrial contamination, and are not indicative of production through any biological system. ALH 84001 Geologic History
Recent observations of the formation history of ALH 84001 point to a complex history of impact deformation, metamorphism, and chemical change, which places further constraints on the presence of biogenic fossils. In the beginning, crystallization of a basaltic magma containing ~2.5–5% interstitialTerm applied to ions or atoms occupying sites between lattice points. Click on Term to Read More melt was followed by contractive deformation and chemical equilibration. A period of aqueous alteration and dehydration of orthopyroxene, resulting in the production of olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More, may have also occurred. Following next was a series of deformations and intense thermal metamorphism, which produced and annealed, respectively, the granular bands. This suggests ALH 84001 was part of a large crater basement in which multiple compression and rarefaction shock waves, approaching 75 gigapascals (GPa), affected the rock. Afterwards, a shock event, with pressures of at least 30 GPa, created fractures that cut across the granular bands and formed feldspathic glass. The next event to occur was the deposition of zoned carbonates (having the morphology of an inverted conic frustum) in the fractures within the granular bands replacing the feldspathic glass (see following photo). A third impact deformation event of ~60 GPa fragmented the carbonate globules and injected melt veins of feldspathic glass into the globules. Evidence for a forth deformation event is also present. This event has reorientated the fragments surrounding a series of fractures, resulting in different magnetic signatures. This event could also represent the launch of ALH 84001 from Mars ~12 m.y. ago. 
Image of a thin sectionThin slice or rock, usually 30 µm thick. Thin sections are used to study rocks with a petrographic microscope. micrograph showing a carbonate globule (white arrow) within a pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More microfracture. The inset shows a close-up of the orange-colored carbonate globule.
Image credit: Ed Scott and Cyrena Goodrich
 Image showing sectioned carbonates exhibiting Oreo-like zoning. Image credit: Ed Scott and Cyrena Goodrich ‘Extraordinary claims require extraordinary evidence.’ –Carl Sagan In a publication by Scott and Barber (2002) and one by Brearley (2002), the authors describe their investigations of magnetite grains embedded within carbonates in ALH 84001, which perhaps represents the most compelling evidence for a biosignature. Employing a transmission electron microscope (TEM), they examined the atomic alignment across the carbonate–magnetite boundary and discovered that the two minerals had identical crystal latticeAtoms or groups of atoms repeated at regular intervals in three dimensions with the same orientation. One may regard each atom or group of atoms as occurring at a point and the resulting collection of points is the space lattice or lattice of the crystal. Click on Term to Read More spacings and orientations in three dimensions, indicating that the embedded magnetite crystal must have formed inside the carbonate crystal. It was argued that the magnetite grains nucleated within nm-scale voids and fractures following the thermal decomposition of Fe–Mg–Ca-carbonate (primarily the more thermally unstable siderite component) into magnetite and carbon dioxide; the voids were created by the loss of this carbon dioxide. Fragmentation and decomposition of the carbonates was probably initiated by a pulse of impact-shock heating (>35 GPa), which occurred ~4 b.y. ago. As such, any possible biogenic magnetite crystals that may have existed in the carbonates prior to the impact would have been altered by the high temperatures (~900°C). It was concluded that all of these spatially-associated magnetite morphologies, including the 27% proposed to be of biogenic origin, were likely formed by the thermal decomposition of carbonates.
Image showing sectioned carbonates exhibiting Oreo-like zoning. Image credit: Ed Scott and Cyrena Goodrich ‘Extraordinary claims require extraordinary evidence.’ –Carl Sagan In a publication by Scott and Barber (2002) and one by Brearley (2002), the authors describe their investigations of magnetite grains embedded within carbonates in ALH 84001, which perhaps represents the most compelling evidence for a biosignature. Employing a transmission electron microscope (TEM), they examined the atomic alignment across the carbonate–magnetite boundary and discovered that the two minerals had identical crystal latticeAtoms or groups of atoms repeated at regular intervals in three dimensions with the same orientation. One may regard each atom or group of atoms as occurring at a point and the resulting collection of points is the space lattice or lattice of the crystal. Click on Term to Read More spacings and orientations in three dimensions, indicating that the embedded magnetite crystal must have formed inside the carbonate crystal. It was argued that the magnetite grains nucleated within nm-scale voids and fractures following the thermal decomposition of Fe–Mg–Ca-carbonate (primarily the more thermally unstable siderite component) into magnetite and carbon dioxide; the voids were created by the loss of this carbon dioxide. Fragmentation and decomposition of the carbonates was probably initiated by a pulse of impact-shock heating (>35 GPa), which occurred ~4 b.y. ago. As such, any possible biogenic magnetite crystals that may have existed in the carbonates prior to the impact would have been altered by the high temperatures (~900°C). It was concluded that all of these spatially-associated magnetite morphologies, including the 27% proposed to be of biogenic origin, were likely formed by the thermal decomposition of carbonates. 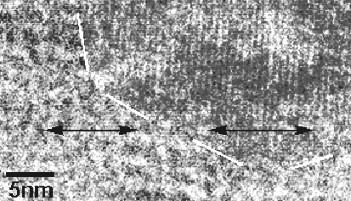
TEM image showing identical alignment between atomic latticeRegularly spaced array of points that represents the structure of a crystal. Crystals are composed of groups of atoms repeated at regular interval in three dimensions with the same orientation. The smallest division of the lattice which can still be used to represent the entire structure is called the unit Click on Term to Read More planes (arrows) of faceted magnetite and host carbonate.
Image credit: Barber and Scott, 2002 Just when it appeared that the case for early biogenic activity on Mars was lost, new evidence for biogenic markers has surfaced. Through the application of the Magnetite Assay for Biogenicity (MAB), K. L. Thomas-Keprta of NASA’s Johnson Space Center, leading an international research team supported by the NASA Astrobiology Institute, reports that 25% of the nanometer-sized magnetites present in carbonates meet the MAB criteria for biogenicity. Additionally, the Mars Global Surveyor has found evidence for a strong ancient magnetic field present on Mars during the time of carbonate formation. These conditions are consistent with the evolution of a magnetotactic bacterial strain similar to the MV-1 type.
Utilizing a scanning electron microscope (SEM), scientists have determined that clusters or colonies of nannobacteria are abundant on Fe–Mg silicates and on a chromite grain studied in ALH 84001, confirming the original statement by McKay et al. in 1996. The present study ruled out the possibility that these objects were artifacts resulting from an excess in gold coating. These nanobodies occur in many shapes (balls, ellipses, ovoids, spheroids, and worm-like), and sizes (20–500 nm, but mainly 30–120 nm). They exhibit no crystal faces, but have rounded shapes consistent with lifeforms rather than mineral crystals. Terrestrial analogs of these nannobacteria having similar dimensions have been identified. It will now be neccesary to determine if these nannobacteria originated on Mars, or are the result of terrrestrial contamination by a previously unknown population of nannobacteria in Antarctica.
Through their continuing studies, Golden et al. (2003) have sought to compare the three-dimensional morphologies of ALH 84001 magnetites to those from the MV-1 bacteria strain and to those produced naturally by the thermal decomposition of organicPertaining to C-containing compounds. Organic compounds can be formed by both biological and non-biological (abiotic) processes. Click on Term to Read More matter, such as by impact shock metamorphismMetamorphism produced by hypervelocity impact between objects of substantial size moving at cosmic velocity (at least several kilometers per second). Kinetic energy is converted into seismic and heat energy almost instantaneously, yielding pressures and temperatures far in excess those in normal terrestrial metamorphism. On planetary bodies with no atmosphere, smaller Click on Term to Read More. In the laboratory they synthesized zoned, Fe-rich carbonate globules similar to those from ALH 84001 through hydrothermal precipitation. Next, they conducted shock experiments to 49 GPa which heated these carbonates to 450+°C and initiated thermal decomposition and the production of magnetite from Mg-rich siderite. The resulting magnetites had similar sizes (~50–100 nm), compositions (100% magnetite to an 80:20 ratio of magnetite and magnesioferrite), and 3-D morphologies to those in ALH 84001, and different from those in MV-1 bacterium, inferring a non-biogenic origin for magnetites in ALH 84001 (Bell 2007).
In contrast to the findings of Golden et al. (2003), which were based on conventional transmission electron microscopy, the team of Thomas-Keprta et al. (2003) utilized electron tomography back-projection techniques to image the nanophase magnetites from both ALH 84001 and MV-1 bacteria. They concluded that the magnetites from both sources were of an equivalent 3-D morphological type—{111}-truncated hexa-octahedral. However, in their independent studies of these magnetites, Golden et al. (2006) found that most do not have the {111}-truncated hexa-octahedral morphology characteristic of biogenic activity, nor are they chemically pure as would be required for a non-decompositional origin postulated by Thomas-Keprta team.
Thereafter, Thomas-Keprta et al. (2008) conducted observational experiments and established thermodynamic models involving the partial thermal decomposition of sideritic carbonates under various heating scenarios. They determined that a vast majority of both chemically pure and impure magnetites present in ALH 84001 carbonates cannot have been produced through thermal decomposition processes, but instead, were introduced into the carbonates from a different source region through some method of transport mechanism. They demonstrated that preferential decomposition of siderite will not produce pure magnetite, and that the magnesite-calcite component of the carbonate would undergo decomposition along with the siderite component, which is not observed (Thomas-Keprta et al., 2009). Furthermore, they determined that graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More would be among the decomposition products under most all oxygen fugacityUsed to express the idealized partial pressure of a gas, in this case oxygen, in a nonideal mixture. Oxygen fugacity (ƒO2) is a measure of the partial pressure of gaseous oxygen that is available to react in a particular environment (e.g. protoplanetary disk, Earth's magma, an asteroid's regolith, etc.) and Click on Term to Read More conditions, but this is also not observed. Perhaps even more telling is the fact that the magnesite layer contained no siderite, and thus could not have decomposed to form magnetite.
A study was conducted by the Carnegie Institution’s Geophysical Laboratory on the macromolecular carbon (MMC) associated with the carbonate globules, and a comparison was made to similar material from the Bockjord VolcanicIgneous rock that forms from cooling magma on the surface of a planet or asteroid. Complex on Svalbard, a territory of Norway. Results were presented at the Astrobiology Science Conference 2006 held in Washington D.C., and it was found that magnetite was always associated with the MMC, and that it likely served as a catalystChemicals that are not consumed in a reaction, but, which speed up the reaction rate. Catalysts aid to form a transition state which is lower in energy than the transition state without the catalyst (essentially decreasing activation energy). Since the barrier to the reaction is lower, the reaction rate increases Click on Term to Read More in the production of MMC—a non-biological synthesis of organic matter on Mars. A similar abioticNon-biological in origin, or not derived from living organisms. Click on Term to Read More origin for the submicron magnetite grains at appropriate temperatures remains most plausible to Treiman and Essene (2011).
and the investigation continues….
The photo shown above is the original NASA photo #S85-39570 showing the complete mass of ALH 84001 as found. Approximately 217 g of this meteorite has been allocated for research. This meteorite is not available to the private collector.
See the PSRD article by Linda M. V. Martel, ‘Did Martian Meteorites Come From These Sources?‘, January 29, 2007.







