Postperovskite
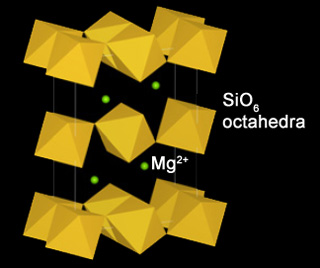
High-pressure form of MgSiO3 with a stacked SiO6-octahedral sheet structure that is a major component of the D” layer in Earth. Unlike perovskiteTerm applied to A2+B4+O3 high-pressure minerals with a perovskite structure (general formula ABX3) where "A" is a metal that forms large cations such as Mg, Fe or Ca, "B" is another metal that forms smaller cations such as Si (called silicate perovskite), Ti and to a lesser degree Al, and Click on Term to Read More, the octahedra formed by oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More ions around the 4+ cationPositively charged ion. Click on Term to Read More have differing orientations. Postperovskite forms from perovskite at conditions exceeding 125 gigapascals and 2500 K, which corresponds to a depth of ~2700 km (near the base of the mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More).The transition results in an increase in densityMass of an object divided by its volume. Density is a characteristic property of a substance (rock vs. ice, e.g.). Some substances (like gases) are easily compressible and have different densities depending on how much pressure is exerted upon them. The Sun is composed of compressible gases and is much Click on Term to Read More of 1.0 to 1.2%. Incorporation of Fe into the structure greatly reduces the pressure needed for the transition. The perovskite-postperovskite phase transition probably produces the D” seismic discontinuity located just above the core-mantle boundary.
***Need references for relationship to meteorites***
Some or all content above used with permission from J. H. Wittke.






