Mantle Phase Changes
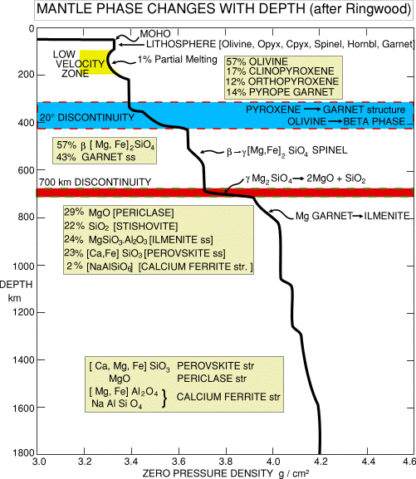
Solid-state mineralogical changes that occur with increasing depth in a planet’s mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More. These are best understood for Earth’s mantle. The change at ~400 km corresponds to a transition from the α- to β-structures of multi-component olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More with ~6% increase in densityMass of an object divided by its volume. Density is a characteristic property of a substance (rock vs. ice, e.g.). Some substances (like gases) are easily compressible and have different densities depending on how much pressure is exerted upon them. The Sun is composed of compressible gases and is much Click on Term to Read More. The small Fe concentration in natural mantle olivine results in a thin region where both phases coexist in equilibriumTerm used to describe physical or chemical stasis. Physical equilibrium may be divided into two types: static and dynamic. Static equilibrium occurs when the components of forces and torques acting in one direction are balanced by components of forces and torques acting in the opposite direction. A system in static Click on Term to Read More and where macroscopically observed density changes occur. Depending actual temperature-depth relationships, the thickness of the two-phase region varies. The ~670-km transformation is fundamentally different from the a-b transition, because it involves a change in chemical composition. The olivine and pyroxene-garnet components transform into magnesiowüstite, (Mg,Fe)O, and perovskiteTerm applied to A2+B4+O3 high-pressure minerals with a perovskite structure (general formula ABX3) where "A" is a metal that forms large cations such as Mg, Fe or Ca, "B" is another metal that forms smaller cations such as Si (called silicate perovskite), Ti and to a lesser degree Al, and Click on Term to Read More with ~10% density increase across the 1-2 km thick phase change region. An addition transformation has been proposed for lowermost 100s of km of the mantle in which perovskite transforms in to a “post-perovskite” phase. Mantle mineralogy and phase changes (after Ringwood for whom ringwooditeHigh-pressure olivine polymorph with a spinel structure that is found in highly shocked meteorites (above ~50 GPa, shock level > S5) and the Earth's transition zone mantle (~13 GPa). Under even higher pressure in the lower mantle (~24 GPa), ringwoodite decomposes into perovskite (Mg,Fe)SiO3, and magnesiowüstite (Mg,Fe)O, whose properties are Click on Term to Read More is named) are shown in the diagram below.
Some or all content above used with permission from J. H. Wittke.






