Auburn

Found: before 1867, Coordinates: 32 ° 37′ N., 85 ° 30′ W., approx.
Formerly considered a probable transported mass of Tombigbee River (IIG)
An iron mass was found in 1859 in western Alabama, USA, followed in subsequent years by the recovery of five additional masses; these six iron masses, named Tombigbee River, had a combined weight of 43.8 kg. In 1867, an extensively oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More 3.63 kg mass was plowed up on the Daniel Plantation located ~250 km east of the Tombigbee River findMeteorite not seen to fall, but recovered at some later date. For example, many finds from Antarctica fell 10,000 to 700,000 years ago. Click on Term to Read More and ~1 mile west of East Alabama College in Auburn. The severely weathered mass of Auburn was broken up with a sledge hammer in a blacksmith’s shop and possibly artificially heated before being described by several different researchers (V. Buchwald, 1975). Although Auburn has historically been considered to be a transported piece of Tombigbee River, it is now demonstrated by Hilton and Walker (2019) to more likely represent a separate iron belonging to the IIAB group. The classification of the Auburn iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More has a long and varied history as described in the following entry from Grady’s Catalogue:
‘A mass of about 8lb (3.63kg) was ploughed up near East Alabama College, C.U. Shephard (1869). Described, with an analysis by O. Hildebrand, 4.67 %Ni, E. Cohen (1905). A second analysis, by A.A. Moss, gave 5.9 %Ni. Classification and analysis, E.R.D. Scott et al. (1973). Description, V.F. Buchwald (1975). Auburn has had a turbulent history, first recognised as an individual meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More, then demoted to a transported piece of Tombigbee River ( q.v._ ). Correspondence between R.S. Clarke Jr., V. Buchwald and J.T. Wasson in 1994 (copies in Min. Dept., NHM, London) has prompted the re-instatement of a Catalogue entry for Auburn. V.F. Buchwald, pers._ commun._ (1994), notes that the octahedral structure and absence of schreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More differentiates Auburn from Tombigbee River, but J.T. Wasson, pers._ commun._ (1994), takes the view that Auburn is a fragment from Tombigbee River. Until the relationship, or otherwise, between Auburn and Tombigbee River is established beyond doubt, then there is more to be gained than lost by keeping separate entries for the two specimens.’
Wasson and Choe (2009) argued that group IIG irons are chemically similar to those of the IIAB iron group, forming extensions to IIAB trends on element–AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More diagrams. It has been proposed by Wasson and Choe (2009) that formation of IIG irons occurred inside isolated cavities which remained after crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More of an evolved IIAB magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More. The IIG irons eventually crystallized in a P-rich region of the lower layer of the IIAB coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More, while an immiscibleThe property of liquids that are mutually insoluble (won't mix together) such as oil and water or metallic and silicate melts. Click on Term to Read More and buoyant S-rich magma collected at the upper regions of the magma chamber. Elements such as Au and Ge were likely removed in the S-rich melt phase, while the low-Ni content of IIG irons is attributed to diffusionMovement of particles from higher chemical potential to lower chemical potential (chemical potential can in most cases of diffusion be represented by a change in concentration). Diffusion, the spontaneous spreading of matter (particles), heat, or momentum, is one type of transport phenomena. Because diffusion is thermally activated, coefficients for diffusion Click on Term to Read More and redistibution of Ni out of metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More and into schreibersite during an extended cooling history. The Ge-isotopic data were obtained by Luais et al. (2014), and they found it to be almost identical for both IIG and IIB metal, while a Ge content of 1.3 ppmParts per million (106). Click on Term to Read More and a δ74Ge of –3.4–° was ascertained for schreibersite in Tombigbee River. Their Ge data support the formation history proposed by Wasson and Choe (2009).
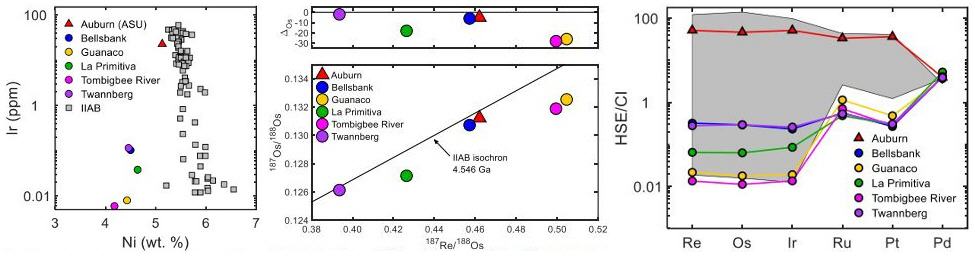
Hilton and Walker (2019) conducted a chemical and isotopic study of each of the IIG irons including a sample of the Auburn mass. They determined that Auburn has a significantly higher Ir concentration than all members of the IIG group, but it is consistent with some IIAB irons. In addition, they demonstrated that IIG and IIAB irons have similarities with respect to their Re–Os isotopic systematics. Furthermore, they found that Auburn has HSE abundances that are different from the IIG irons, but are consistent with some IIAB members such as Coahuila. These data suggest a likely genetic relationship between Auburn and the IIAB group irons, and they plan to use Mo isotopes in future studies to determine whether or not a genetic connection exists between the IIG and IIAB group irons (see diagrams below).
The 13.3 g angular specimen pictured above is a corroded fragment with label provenance from the Auburn mass, previously part of the Thomas M. Bee Collection. Most specimens of the Auburn meteorite are similar in size to this one or smaller, consisting of small angular fingers of kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More after undergoing significant terrestrial corrosion and disintegration over the intervening years. The photo below shows two large fragments of Auburn weighing 1.7 and 0.7 kg

.






