Iron, IIG
(Compositionally linked to group IIAB)
Found 1859
32° 14′ N., 88° 12′ W. The Tombigbee River meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More comprises a number of masses that fell over a 14 km ellipse aligned almost in a north–south direction (the largest masses to the south) in Choctaw County, near Jachin, Alabama. Although a meteorHow long Sonic booms Of the several 10s of tons of cosmic material entering Earth's atmosphere each day, only about one ton reaches the surface. An object's chance of survival depends on its initial mass, speed and angle of entry, and friability (tendency to break up). Micrometeoroids radiate heat so Click on Term to Read More that was seen over most of Alabama in early 1848 was reported in newspapers from cities in the region of Jachin, the high level of oxidationOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More exhibited by the Tombigbee iron masses make an association doubtful. The first of these masses, weighing 757 g, was discovered in 1859 by Mr. Ben Johnson. This first mass, along with five of the larger masses weighing ~ 15.0, 12.0, 9.2, 3.6, and 3.3 kg, were sold to the mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More dealer A. E. Foote in 1899.
Around this time, an additional ~3.6 kg mass was plowed up on the Daniel Plantation, one mile from the East Alabama Male College, Auburn. Due to the close similarities in chemical composition, structure, and weathering degree between Auburn and Tombigbee River, it was generally accepted that Auburn is a transported mass of Tombigbee River. The differences that do exist between the two irons—notably, the octahedral structure and the absence of
schreibersiteNi-Fe phosphide mineral, (Fe,Ni)3P, yellowish in color and predominantly found in iron and stony-iron meteorites. Schreibersite can also be found in a variety of other meteorites including some acapulcoites, aubrites, enstatite chondrites and achondrites, lunars, ureilites, winonaites and a smattering of other meteorite types like CM, CO and CB. Schreibersite Click on Term to Read More in Auburn—are consistent with the heterogeneous nature of this meteorite. Nevertheless, through correspondence between R.S. Clarke Jr., V.F. Buchwald, and J.T. Wasson in 1994 (copies in Min. Dept., NHM, London), it was decided that it was best to maintain a separate catalog entry for Auburn. Recent analyses of the Auburn mass (#443) in the Carleton B. Moore Meteorite Collection (Arizona State University) have demonstrated that this is likely a separate iron belonging to the chemical group IIAB (see the
Auburn page for more complete details).
Tombigbee River is the most phosphorus-rich meteorite known, with abundant low-Ni schreibersite ribbons accounting for 9–15% by area. This high P content is consistent with late
crystallizationPhysical or chemical process or action that results in the formation of regularly-shaped, -sized, and -patterned solid forms known as crystals. Click on Term to Read More of dense, P-rich
magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More pockets following large degrees of
fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More (>80%). Group IIG members are chemically most similar to those of the IIAB iron group, forming extensions to IIAB trends on element–
AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More diagrams. It has been proposed by Wasson and Choe (2009) that formation of IIG irons occurred inside isolated cavities which remained after crystallization of an evolved IIAB magma. The IIG irons eventually crystallized in a P-rich region of the lower layer of the IIAB
coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More, while an
immiscibleThe property of liquids that are mutually insoluble (won't mix together) such as oil and water or metallic and silicate melts. Click on Term to Read More and buoyant S-rich magma collected at the upper regions of the magma chamber. Elements such as Au and Ge were likely removed in the S-rich melt phase, while the low-Ni content of IIG irons is attributed to
diffusionMovement of particles from higher chemical potential to lower chemical potential (chemical potential can in most cases of diffusion be represented by a change in concentration). Diffusion, the spontaneous spreading of matter (particles), heat, or momentum, is one type of transport phenomena. Because diffusion is thermally activated, coefficients for diffusion Click on Term to Read More and redistibution of Ni out of
metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More and into schreibersite during an extended cooling history. The Ge-isotopic data were obtained by Luais
et al. (2014), and they found it to be almost identical for both IIG and IIB metal, while a Ge content of 1.3
ppmParts per million (106). Click on Term to Read More and a δ74Ge of –3.4‰ was ascertained for schreibersite in Tombigbee River. Their Ge data support the formation history proposed by Wasson and Choe (2009).
Although Tombigbee River was initially classified as an
ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More iron, it shared a similar composition with two other iron meteorites, Bellsbank and La Primitiva. As such, these three meteorites became known as the Bellsbank Trio. A fourth member of this iron grouplet, Twannberg, was subsequently recovered and the grouplet became known as the Bellsbank Quartet. In the year 2000, a fifth member of this grouplet, Guanaco, was recovered in the Atacama Desert, Chile, providing the requisite number of members needed to establish a new iron chemical group—
The Bellsbank Quintet. John T. Wasson therefore proposed that this new iron group be designated IIG, its members representing the most Ni-poor (4.3%), schreibersite-rich meteorites of all iron groups. It is noteworthy that a sample of an
iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More from China, named Wu-Chu-Mu-Ch’in, which has a heterogeneous chemical composition and structure, includes a portion with trace elements that plot within the Bellsbank group IIG (Bartoschewitz, 2003). This iron portion also has the lowest Ni content (3.5%) of any known iron meteorite.
Most specimens in this group have a hexahedrite-like
matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More consisting predominately of
kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More, but one Tombigbee River mass, which is lower in P and higher in Ni, shows a small area with a remnant coarse Thomson (Widmanstätten) structure. It was proposed by Vagn F. Buchwald that these irons be structurally classified as
hexahedriteOne of the main types of iron meteorites composed almost entirely of kamacite and named for its cubic (hexahedral) cleavage of α-Fe-Ni crystals. Upon etching, hexahedrites do not display a Widmanstätten pattern, but do often exhibit fine, parallel lines called Neumann lines for their discoverer, Franz Neumann, who first studied Click on Term to Read More, transitional to coarsest
octahedriteMost Common type of iron meteorite, composed mainly of taenite and kamacite and named for the octahedral (eight-sided) shape of the kamacite crystals. When sliced, polished and etched with an acid such as nitric acid, they display a characteristic Widmanstätten pattern. Spaces between larger kamacite and taenite plates are often Click on Term to Read More (H–Ogg). The masses have been terrestrially corroded but rare
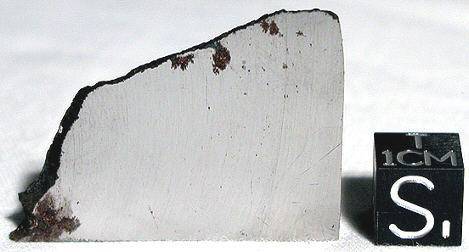
fusion crustMelted exterior of a meteorite that forms when it passes through Earth’s atmosphere. Friction with the air will raise a meteorite’s surface temperature upwards of 4800 K (8180 °F) and will melt (ablate) the surface minerals and flow backwards over the surface as shown in the Lafayette meteorite photograph below. Click on Term to Read More and heat-affected zones are still visible. Neumann bands record more than one deformation event including the violent entry into Earth’s atmosphere. The photo above is a 6.64 g partial slice from a Tombigbee River specimen.







