r-process
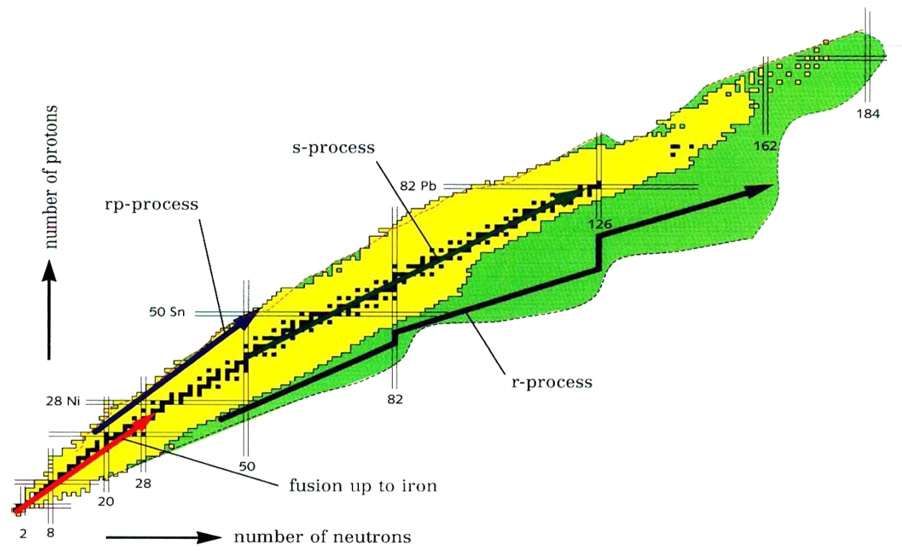
Rapid (hence “r”) absorptionTransfer of energy to a medium as a particle or electromagnetic radiation passes through it. Absorption of electromagnetic radiation is the combined result of Compton scattering, σ, and photoelectric absorption, τ. It may be quantified: where, t = thickness, ρ = density, and μ = mass absorption coefficient, which combines Compton and photoelectric effects (μ = σ + τ). of neutrons by atoms when the neutron flux is very high (~1022 neutrons per cm2/s) and the temperature is very high (T > 109 K). These conditions are hypothesized to occur during a supernovaStellar explosion that expels much or all of the stellar material with great force, driving a blast wave into the surrounding space, and leaving a supernova remnant. Supernovae are classified based on the presence or absence of features in their optical spectra taken near maximum light. They were first categorized explosion/collapse or neutron starDense ball of neutrons that remains at the core of a star after a supernova explosion has destroyed the rest of a star with mass 8-18 (?) Msun. A neutron star has mass ~2-3 Msun, density ~1014 g/cm3, and is supported by neutron degeneracy pressure. Typical neutron stars are 10-20 mergers. The time between neutron captures is much shorter than the average b decay half-lifePeriod of time required for 50% (½) of the atoms of a radioactive nuclide in a sample to decay. After two half-lives, 25% ( ½ x ½ = 1/4) of the original radioactive nuclide will remain. After three half-lives, 12.5% ( ½ x ½ x ½ = 1/8) of the original radioactive nuclide will remain. (on the order of 0.1 to 1 seconds) of these neutron-rich nuclei. Capture moves the nucleusCore of an atom, where nearly the entire mass and all positive charge is concentrated. It consists of protons and neutrons. toward “neutron drip line” where the probability for absorbing a new neutron is overwhelmed by the probability that a neutron will be knocked off by photodisintegration. This balance point defines the (n, γ) ↔ (γ, n) equilibriumTerm used to describe physical or chemical stasis. Physical equilibrium may be divided into two types: static and dynamic. Static equilibrium occurs when the components of forces and torques acting in one direction are balanced by components of forces and torques acting in the opposite direction. A system in static. The path of nucleosynthesis moves up along a line somewhere between the valley of stability and the neutron drip line (the offset depending on conditions such as temperature, neutron flux, and photonDiscrete bundle of light energy. Light of a given energy (frequency) cannot be broken up indefinitely. Rather for a given frequency it comes in discrete bundles with energy (h = Planck's constant and ν= frequency): It is often useful to think of light as a bunch of particle photons; whereas, flux) until finally fissionBreaking apart of a body into smaller fragments. In nuclear physics, fission refers to splitting of a heavy atomic nucleus into two or more lighter nuclei with an associated release of energy. The mass of the nucleus before fission is greater than the combined masses of the resulting fragments; the blocks the chain in the actinide region. Nuclei with “magic” neutron numbers serve as bottlenecks to nuclei climbing the r-process path. For example, 130Cd is an isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: H, H (deuterium), and H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. with the A = 82 magic number, but the heaviest stable isotope of cadmium is 116Cd with 14 fewer neutrons.
If the neutron source only lasts for a short time, highly unstable nuclei will be left on the r-process path, with many stuck at the “magic” bottlenecks. These undergo b decay back to the line of stability. In our example, 130Cd would eventually decay to 130Te, the most abundant isotope of tellurium. Since β decay reduces the number of neutrons, abundance peaks show up at lower neutron number than the s-processSlow neutron capture by nuclei in massive stars. In the s-process, one starts with existing iron-group nuclei. Therefore, it would only be expected to take place in second-generation stars that collapsed out of the residue of a previous supernova explosion. The flux of neutrons is small enough that rate of peaks.
In some cases, the r-process may be fast enough to break through the region of α-instability beyond 208Pb. The stable actinidesElements Ac (actinium) to Lr (lawrencium) found in the bottom row of the inner-transition elements of the periodic table. These elements are all radio active and the heavier in the series are unstable. For these elements the 5f orbital is the filling orbital. may be produced directly from a neutron-rich precursor, or from α-decay of even heavier elements.
The r-process is one of three nucleosynthesis processes that also includes the s-process and the rp-processRapid proton capture (hence "rp") is a process that synthesizes elements by successive proton absorption and β+ decay; thus, it tracks somewhere between the valley of stability. The rp-process is one of three nucleosynthesis processes that also includes the s-process and the r-process..
Some or all content above used with permission from J. H. Wittke.






