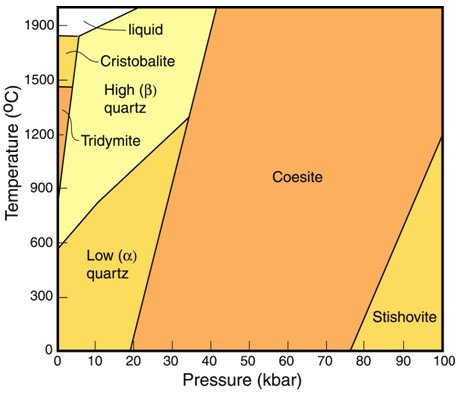
High-Pressure Mineral Phases
MineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More forms that are stable only at the extremely high pressures typical of Earth’s deep interior, but not its surface. Such pressures also are generated instantaneously during meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More impact. For example, coesiteHigh-pressure polymorph of silicon dioxide (SiO2). Has the same chemical composition as cristobalite, stishovite, seifertite and tridymite but possesses a different crystal structure. Coesite forms at intense pressures of above about 2.5 GPa (25 kbar) and temperature above about 700 °C, and was first found naturally on Earth in impact Click on Term to Read More and stishoviteDense, high-pressure phase of quartz; so far identified only in shock-metamorphosed, quartz-bearing rocks from meteorite impact craters. Stishovite was synthesized in 1961 before it was discovered at Meteor Crater, Arizona. Its structure consists of parallel chains of single SiO6 octahedra. The octahedra are on their sides, sharing opposing edges. Image Click on Term to Read More are high-pressure polymorphs of SiO2 (silicaSilicon dioxide, SiO2.) and diamondOne of the naturally occurring forms of carbon found in meteorites. Each C atom is bonded through covalent sp3 hydrid orbitals to four others. The strength of the C-C bonds makes diamond the hardest naturally occurring substance (according to the Mohs scale) in terms of resistance to scratching. There are Click on Term to Read More is a high-pressure modification of graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More (carbonElement commonly found in meteorites, it occurs in several structural forms (polymorphs). All polymorphs are shown to the left with * indicating that it been found in meteorites and impact structures: a. diamond*; b. graphite*; c. lonsdalite*; d. buckminsterfullerene* (C60); e. C540; f. C70; g. amorphous carbon; h. carbon nanotube*. Click on Term to Read More).
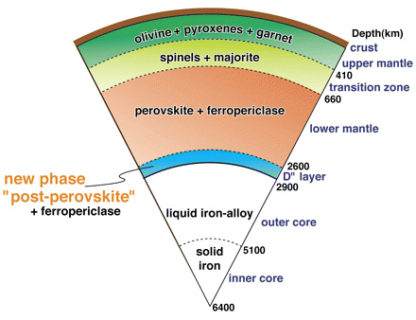
The minerals in mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More rocks undergo phase changes with depth, transforming into denser forms. Particularly notable are the transformations that occur near 400 and 700 km depth. The former corresponds to pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More transforming into a garnetMineral generally found in terrestrial metamorphic rocks, although igneous examples are not uncommon. Garnet is a significant reservoir of Al in the Earth's upper mantle. The garnet structure consists of isolated SiO4 tetrahedra bound to two cation sites. The A site holds relatively large divalent cations (Ca2+, Mg2+, Fe2+, Mn2+); the Click on Term to Read More structure.
Some or all content above used with permission from J. H. Wittke.






