Amphibole Group
 Complex family of hydrous double-chain inosilicate minerals. Amphiboles are common in terrestrial metamorphicRocks that have recrystallized in a solid state due to changes in temperature, pressure, and chemical environment. Click on Term to Read More and igneous rocks, forming in the presence of water. Except for kaersutite amphibole that is commonly found in Martian shergottitesIgneous stony meteorite with a Martian origin consisting mainly of plagioclase (or a shocked glass of plagioclase composition) and pyroxene. They are the most abundant type of SNC meteorites and the type member is the Shergotty meteorite, which fell in India in 1865. Shergottites are igneous rocks of volcanic or Click on Term to Read More, amphiboles in general are not often found in extraterrestrial materials.The first report of meteoritic amphibole was in April 1967 when Richard Olson announced that the amphibole richterite (soda tremolite), Na2Ca(Mg, Fe)5Si8O22(OH, F)2, had been discovered within graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More nodules in the Wichita County iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More. In 1971, he also reported finding richterrite in the Abee (EH4) and Canyon Diablo (Iron, IAB-MG iron) meteorites.
Complex family of hydrous double-chain inosilicate minerals. Amphiboles are common in terrestrial metamorphicRocks that have recrystallized in a solid state due to changes in temperature, pressure, and chemical environment. Click on Term to Read More and igneous rocks, forming in the presence of water. Except for kaersutite amphibole that is commonly found in Martian shergottitesIgneous stony meteorite with a Martian origin consisting mainly of plagioclase (or a shocked glass of plagioclase composition) and pyroxene. They are the most abundant type of SNC meteorites and the type member is the Shergotty meteorite, which fell in India in 1865. Shergottites are igneous rocks of volcanic or Click on Term to Read More, amphiboles in general are not often found in extraterrestrial materials.The first report of meteoritic amphibole was in April 1967 when Richard Olson announced that the amphibole richterite (soda tremolite), Na2Ca(Mg, Fe)5Si8O22(OH, F)2, had been discovered within graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More nodules in the Wichita County iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More. In 1971, he also reported finding richterrite in the Abee (EH4) and Canyon Diablo (Iron, IAB-MG iron) meteorites.
The amphibole structure consists of doubled Si4O11 chains running parallel to c-axis. These chains are bonded to octahedral strips consisting of three regular octahedral sites (M1, M2, M3) and one larger 6- to 8-fold site (M4). In addition there is an even larger 10- to 12-fold A site that is usually empty.
OH– groups occur in the interiors of the rings in the double chains.
The result is an “I-beam” structure like that of pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More. The M123 cations are coordinated by oxygens and OH- groups of the adjacent double chains, forming a TOT strip. Amphibole TOT strips are approximately twice as wide as pyroxene strips, yielding typical near 120° {110} cleavage.
Amphiboles are composed of the same cations as pyroxenes, but also have OH- groups, resulting in lower densitiesMass of an object divided by its volume. Density is a characteristic property of a substance (rock vs. ice, e.g.). Some substances (like gases) are easily compressible and have different densities depending on how much pressure is exerted upon them. The Sun is composed of compressible gases and is much Click on Term to Read More and refractive indices than their pyroxene counterparts. Their general formula is A0-1X2Y5Z8O22(OH,F,Cl)2. The A site holds large cations such as Na+ and K+ and is commonly not completely filled. The X site (M4) holds a large to intermediate-size cations such as Ca2+, Na+, Mn2+, Fe2+, Mg2+, and Li+. The Y sites (M1, M2, M3) hold intermediate-sized to small cations such as Mn2++, Fe2+, Mg2+, Fe3+, Al3+, and Ti4+. Lastly, the tetrahedral Z sites in the chains hold the smallest cations Si4+ and Al3+. As indicated by the formula, substitutionReplacement of one ion or ionic group for another in the same structural site in a mineral yielding a solid solution. Most substitution in minerals is of cations which are smaller and essentially sit in a lattice of oxygen anions. Anionic substitution does occur in halides. Substitutions are classified based of F– and Cl– can occur for HO–.
The cationPositively charged ion. Click on Term to Read More substitutions that produce the wide range of amphibole compositions can be complex. Multiple coupled substitutions are usually required to maintain charge balance. Simple cation substitutions include:
- Na+ ↔ K+ (A)
- Ca2+ ↔ Mg2+ ↔ Fe2+ (M4)
- Mg2+ ↔ Fe2+ and Fe3+ ↔ Al3+ (M123)
Coupled substitutions include:
- Mg2+,Fe2+)M123 + (Si4+)T ↔ (Fe3+,Al3+)M123 + (Al3+)T
- ( )A + (Si4+)T ↔ (Na+)A + (Al3+)T
- (Ca2+)M4 ↔ (Na+)A + (Na+)M4
- 2(Ca2+)M4 + (Mg2+,Fe2+)M123 ↔ (Na+)A + 2(Na+)M4 + (Al3+)M123
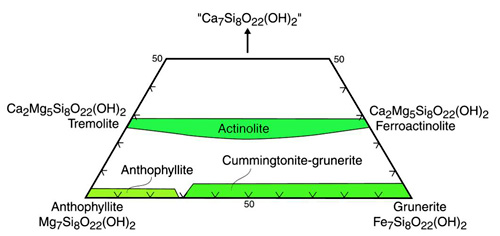
Classification of Ca-rich amphiboles is done in a manner analogous to that for quadrilateral pyroxenes by plotting the Ca, Mg, and Fe end-member components. No amphiboles plot above the above 2/7th Ca line when both M4 sites completely filled with Ca.
Cation substitutions complicate the situation. The most common amphibole, hornblende, has very variable composition owing to significant substitution of Na+ and K+ in A site and Fe3+ and Al3+ in M123 sites. Hornblende is the result of two main substitutions starting from tremolite: Si4+ + (Mg2+,Fe2+) ↔ 2Al3+, and Si4+ + A-site ↔ Al3+ + (Na+,K+). Hornblende includes following series: magnesiohornblende-ferrohornblende, tschermakite-ferrotschermakite, edenite-ferroedenite, pargasite-ferropargasite, and magnesiohastingsite-hastingsite (mainly listed to show the spectacular mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More names). One way of classifying hornblende is to consider the A-site occupancy and the amount of Si in the T-site. In the diagram below yellow shows the range of observed composition with orange indicating the more abundant ones (Hb = hornblende (sensu stricto), Ts = tschermakite, Ed = edenite, and Pa = pargasite).
There are alkali-rich varieties in addition to the more common calcic amphiboles. Alkali amphibole occurs primarily in sodic-metamorphic rocks and in alkaline igneous rocks. Alkali amphiboles are divided into two groups based upon M4 and A site occupancies. If the M4 site contains <0.5 (Na+K) and there is no Na in the A site, the mineral is considered a low-Na alkali amphibole. If the M4 site contains >0.5 (Na+K) and there is Na in the A site, it is considered a high-Na alkali amphibole. There is no solid solutionCompositional variation resulting from the substitution of one ion or ionic compound for another ion or ionic compound in an isostructural material. This results in a mineral structure with specific atomic sites occupied by two or more ions or ionic groups in variable proportions. Solid solutions can be complete (with between calcic and alkali amphiboles. The two types of alkali amphiboles are further subdivided. Classification of low-Na alkali amphiboles is accomplished using the diagram below; the most common types are glaucophane and riebeckite.
Classification of the rarer high-Na alkali amphiboles is done with the diagram below.
A summary of amphibole chemistry is given below (green = calcic amphiboles; blue = alkali amphiboles).
Some or all content above used with permission from J. H. Wittke.