Beta Decay
Nuclear decays which proceed via the weak interaction are beta (β) decays. These include all nuclear decays in which the atomic massMass of a neutral atom of a nuclide - also called "atomic weight." The atomic weight of an element is the weighted average of each isotope. (A) remains constant and the atomic numberA number equivalent to the number of protons in the nucleus of an atom, commonly abbreviated as Z., Z, changes by one unit. Examples of β decay processes include:

The most elementary β decay process is free neutronCharge-neutral hadron with a mass of 1.6748 x 10 kg, equivalent to 939.573 MeV, and an intrinsic angular momentum, or spin, of ½ (in units of h/2π). The neutron is a nucleon, one of the two basic constituents of all atomic nuclei (apart from H, which consists of a single decay:
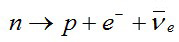
“Beta” originally referred to electrons, but β decays can involve electrons or positrons, and either electron neutrinos or anti-neutrinos.
Some or all content above used with permission from J. H. Wittke.






