Methods for Dating the Age of Meteorites
Methods of Dating the Age of Meteorites
Meteorites are among the oldest objects we know about – formed about 4.5 billion years ago. But how do scientists know this? This article describes the principles and methods used to make that determination.There are well-known methods of finding the ages of some natural objects. Trees undergo spurts in growth in the spring and summer months while becoming somewhat dormant in the fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More and winter months. When a tree is cut down, these periods are exhibited in a cross section of the trunk in the form of rings. Simply counting the number of rings will give one a fairly good idea of the age of the tree. Periods of heavy rain and lots of sunshine will make larger gaps of growth in the rings, while periods of drought might make it difficult to count individual rings. When determining the ages of very old objects, the only suitable clocks we have found involve the measurement of decay products of radioactive isotopes. Isotopes are atoms of the same elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More with different amounts of neutrons. Some isotopes are stable, whereas others are radioactive and decay into other components called daughter isotopes. For example, hydrogenLightest and most common element in the universe (~92% by atoms; ~75% by mass). Hydrogen's isotopes are: • 1H (99.9885 %)
• 2H (0.0115 %), also called deuterium.
• 3H, also called Tritium, is a radioactive (t½ = 12.32 y) by-product of atmospheric thermonuclear tests in Earth's hydrosphere and atmosphere.
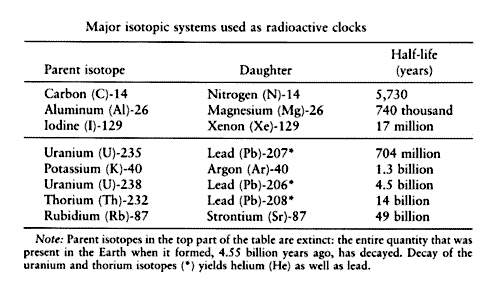
Click on Term to Read More has two stable isotopes 1H (ordinary hydrogen), 2H (deuteriumAlso called heavy hydrogen, deuterium is an isotope of hydrogen (D, or 2H) whose nucleus contains one proton and one neutron. As a trace element formed during the nucleosynthesis epoch of the Big Bang, deuterium is an important indicator of the baryon density in the universe. The larger the density, the Click on Term to Read More), and one radioactive isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More 3H (tritium). The superscript denotes the atomic weightMass of a neutral atom of a nuclide - also called "atomic weight." The atomic weight of an element is the weighted average of each isotope. Click on Term to Read More of the isotope (the number of protons and neutrons). Radioactive isotopes decay according to a power law, and the typical unit given for this is called the half-lifePeriod of time required for 50% (½) of the atoms of a radioactive nuclide in a sample to decay. After two half-lives, 25% ( ½ x ½ = 1/4) of the original radioactive nuclide will remain. After three half-lives, 12.5% ( ½ x ½ x ½ = 1/8) of the original radioactive nuclide will remain. Click on Term to Read More of the isotope. When a given quantity of an isotope is created (in a supernovae, for example), after the half-life has expired, 50% of the parent isotope will have decomposed into daughter isotopes. After the second half-life has elapsed, yet another 50% of the remaining parent isotope will decay into daughter isotopes, and so on. For all practical purposes, the original isotope is considered extinct after 6 half-life intervals. Some of the isotopes and their daughters are shown in the following table (from Dodd):
87Srnow = 87Sroriginal + (87Rboriginal – 87Rbnow)
The term in parenthesis, the amount of 87Rb that decayed into 87Sr can be related by the radioactive decayProcess in which an isotope's nucleus changes ('decays') to produce another isotope. The original atom is called the 'parent' and the resulting atom, the 'daughter'. There are three modes of radioactive decay: • Emission of a particle (He nucleus) that decreases the atomic number (Z) by 2 and the atomic Click on Term to Read More law:
87Rboriginal = 87Rbnow * (elt)
where, e is the base of the natural logarithm,
l is the rate of radioactive decay,
and t is the elapsed time.
87Srnow = 87Sroriginal + 87Rbnow * (elt – 1)
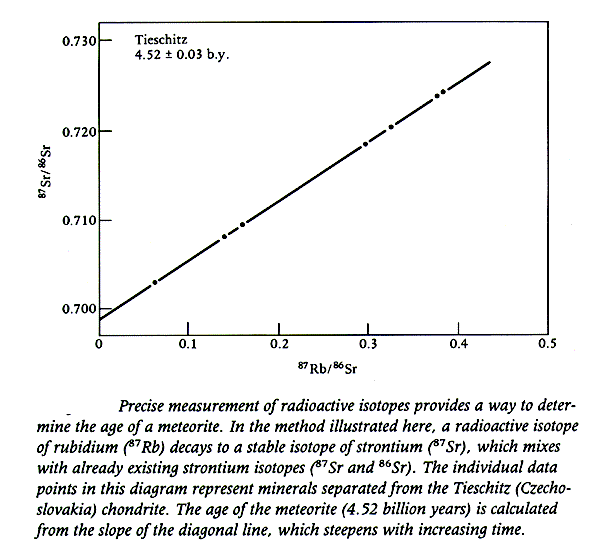
Along with 87Sr, 86Sr also occurs in meteorites, but it is not a decay product and its amount does not change over time, so we can divide this constant in the above equation without changing the equality:
87Srnow / 86Sr = 87Sroriginal / 86Sr + 87Rbnow / 86Sr * (elt – 1)
Note that this is the equation of a line in the form
y = mx + b
where, m, the slope, is (elt – 1)
and b, the y intercept, is the original strontium isotope ratio.
How are these Measured?
Scientists use a mass spectrometerInstrument used to measure the masses of molecules and atoms by volatilizing and then ionizing them. The ions are then separated magnetically according to their mass-to-charge ratio in order to determine the chemical or isotopic composition of a substance. Click on Term to Read More to obtain these ratios. A small portion of a meteorite is vaporized in the device forming ions. These ions are accelerated in an electric field through collimating slits and subject to a magnetic field which causes the ions to follow a curved path. The ions are deflected according to their mass. By adjustment of the strength of the magnetic field and suitable placement of an ionAtom with a net electrical charge because it has lost, or gained, one or more electrons relative to the number possessed by a neutral atom of the same element. A positively charged ion (cation) has fewer electrons than a neutral atom; a negatively charged ion (anion) has more. Click on Term to Read More collector, the different isotopes can be measured with precision. Complications There are some things that affect these measurements. Thermal processes that may occur during meteorite impact in the lifetime of the specimen can reset some of the atomic clocks, mixing components and releasing important gases such as 129Xe and 40Ar. In practice, several isotope systems and several samples are used to determine the ages. Meteorites that are mostly unaltered (petrological type 3) serve as the best samples. Epilog: Jan. 24, 1999 I received private communications from scientists about this paper, which was based mainly on work done in the 1980’s. Nowadays, 146Sm – 142Nd with a mean life of 1.49 x 108 years is also used, along with other methods to date meteorites. In one note, from Dr. Bogard at NASA, it was mentioned to me that:‘You refer to extinct nuclides 14C, 26Al, and 129I. Only the latter two ‘extinct’ nuclides are used in dating. The use of 14C in meteorite dating is solely based on its production by cosmic raysHigh-energy subatomic particles mainly originating outside the Solar System that continuously bombard the Earth from all directions. They represent one of the few direct samples of matter from outside our solar system and travel through space at nearly the speed of light. These charged particles – positively charged protons or Click on Term to Read More (and for terrestrial samples, with its production in the atmosphere). 26Al and some other nuclides not mentioned are also used in this way. Thus, although ‘extinct’, these nuclides are present in meteorites, but produced by a more recent process. ‘The idea that Rb-Sr is the most used chronometer for meteorites is largely based on work done 10-30 years ago. Increasingly, the other techniques are used, such that probably no one technique dominates for meteorite dating. Rb-Sr is a good example for explaining the process, however. ‘Near the end you imply that low petrologic typeMeasure of the degree of aqueous alteration (Types 1 and 2) and thermal metamorphism (Types 3-6) experienced by a chondritic meteorite. Type 3 chondrites are further subdivided into 3.0 through 3.9 subtypes. chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More are the most easily dated. Actually, meteorites that formed by melting, e.g., the various types of achondrites, usually give more precise ages. Type-3 chondrites can contain phases with slightly different ages, and some phases have been slightly altered by parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More processes.’
References
Meteorites and Their Parent Planets, Harry Y. McSween, Cambridge University Press, 1987. Thunderstones and Shooting Stars, Robert T. Dodd, Harvard University Press, 1986. Created on 25 Sep 1998 by Jim Hurley
For additional information on radiometric dating, read the PSRD article by Alexander N. Krott: Dating the Earliest Solids in our Solar System , Sep 2002. Another PSRD article, Using Aluminum-26 as a Clock for Early Solar System Events , Sep 2002, by Ernst Zinner, examines how Al-26 can be used as a fine-scale chronometer for early solar systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with events. A detailed description of radiometric dating using the isochron method can be found on the website of Chris Stassen.






