Iron, ungroupedModifying term used to describe meteorites that are mineralogically and/or chemically unique and defy classification into the group or sub-group they most closely resemble. Some examples include Ungrouped Achondrite (achondrite-ung), Ungrouped Chondrite (chondrite-ung), Ungrouped Iron (iron-ung), and Ungrouped Carbonaceous (C-ung). Click on Term to Read More plessitic octahedriteMost Common type of iron meteorite, composed mainly of taenite and kamacite and named for the octahedral (eight-sided) shape of the kamacite crystals. When sliced, polished and etched with an acid such as nitric acid, they display a characteristic Widmanstätten pattern. Spaces between larger kamacite and taenite plates are often Click on Term to Read More
Found 2000
no coordinates recorded Many individual masses from this rare shower-producing meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More have been recovered in Taza, Morocco, having a total weight of 75.3 kg. This meteorite has been assigned number 859 in the NWA series. Many of the Taza masses have well-oriented shapes.
Taza is chemically anomalous and unrelated to any established iron chemical group. However, Taza has an elemental composition very similar to the ungrouped, plessitic iron, Butler, and the two can be clearly grouped together (see table below). They have an extremely high Ge concentration of 2000–2300
ppmParts per million (106). Click on Term to Read More (Wasson, 2011), four times higher than any other
iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More. Similarly, their
AuThe astronomical unit for length is described as the "mean" distance (average of aphelion and perihelion distances) between the Earth and the Sun. Though most references state the value for 1 AU to be approximately 150 million kilometers, the currently accepted precise value for the AU is 149,597,870.66 km. The Click on Term to Read More and As contents are high. A high Ni content of ~16% promotes
kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More to form discontinuous, pointed spindles, rimmed by
taeniteLess common than kamacite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Taenite, γ-(Fe,Ni), has 27-65 wt% Ni, and forms small crystals that appear as highly reflecting thin ribbons on the etched surface of a meteorite; the name derives from the Greek word for "ribbon." Click on Term to Read More, with widths measuring ~0.15 mm. Tetrataenite (~50% Ni) forms a narrow border on some kamacite spindles. In the dense, fine-grained
plessiteA fine-grained intergrowth of kamacite and taenite that fills in the wedges between wide kamacite and taenite bands in octahedrites. The name derives from the Greek word for "filling." Click on Term to Read More matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More, this spindle pattern is repeated on a scale ten times finer to form a µm-sized Thomson (Widmanstätten) structure. This uncommon plessitic microstructure is transitional between the octahedrites and the ataxites.
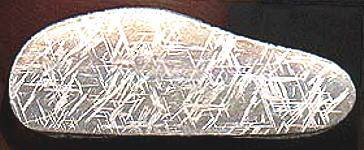
It has been tentatively declared that Taza and Butler are probably nonmagmatic irons (Wasson, 2011). The specimen of Taza shown above is a 13.42 g etched partial slice that displays kamacite spindles in a Thomson (Widmanstätten) pattern.
Comparison of VolatileSubstances which have a tendency to enter the gas phase relatively easily (by evaporation, addition of heat, etc.). ElementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More Abundances for Taza and Butler
|
Co(%) |
Ni(%) |
Cu(ppm) |
Ga(ppm) |
Ge(ppm) |
As(ppm) |
W(ppm) |
Ir(ppm) |
Pt(ppm) |
Au(ppm) |
| Taza |
1.29 |
16.34 |
291 |
87.2 |
2290 |
54 |
6.7 |
2.42 |
38 |
6.48 |
| Butler |
1.21 |
15.67 |
151 |
87.1 |
1970 |
48 |
5.1 |
1.8 |
34.9 |
6.77 |







